Cells of the Maternal-Fetal Interface May Contribute to Epidural-Related Maternal Fever After Administration of Ropivacaine: The Role of Phosphatases DUSP9 and PHLPP1
- PMID: 40564984
- PMCID: PMC12193418
- DOI: 10.3390/ijms26125520
Cells of the Maternal-Fetal Interface May Contribute to Epidural-Related Maternal Fever After Administration of Ropivacaine: The Role of Phosphatases DUSP9 and PHLPP1
Abstract
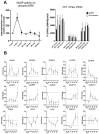
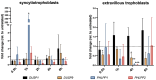
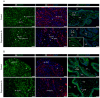
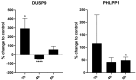
Epidural-related maternal fever (ERMF) occurs with significant incidence in women receiving local anesthetics such as ropivacaine via epidural catheter for pain relief during labor. The causal mechanism behind this phenomenon is still not fully resolved, but evidence suggests that these anesthetics cause sterile inflammation. In this observational study, we investigated a possible contributory role of the dual-specificity phosphatase-9 (DUSP9) controlling the activity of mitogen-activated protein kinases (MAPK), and also PH-domain and Leucine-rich repeat phosphatase (PHLPP) regulating AKT kinases. The data show that ropivacaine differentially affects the expression of these phosphatases in distinct cell types of the umbilical cord and placenta. The gene expression of DUSP9 was almost completely switched off in the presence of ropivacaine in HUVECs and extravillous trophoblasts for up to 6 h, while the expression of PHLPP1 was upregulated in HUVECs and syncytiotrophoblasts. Extravillous trophoblasts were identified as a source of pro-inflammatory mediators and regulatory miRNAs in response to ropivacaine. Placentae at term exhibited a distinct DUSP9 expression pattern, whether the patients belonged to the control group or received epidural analgesia with or without elevated body temperature. The observed data imply that ropivacaine induces complex effects on the MAPK and AKT pathways at the feto-maternal interface, which contribute to the ERMF phenomenon.
Keywords: DUSP9; ERMF; PHLPP1; dual-specificity phosphatase; epidural analgesia; epidural-related maternal fever.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

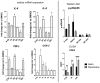
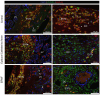
References
-
- Wohlrab P., Boehme S., Kaun C., Wojta J., Spittler A., Saleh L., Knofler M., Markstaller K., Klein K.U., Tretter V. Ropivacaine Activates Multiple Proapoptotic and Inflammatory Signaling Pathways That Might Subsume to Trigger Epidural-Related Maternal Fever. Anesth. Analg. 2020;130:321–331. doi: 10.1213/ANE.0000000000004402. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

