Oleocanthal as a Multifunctional Anti-Cancer Agent: Mechanistic Insights, Advanced Delivery Strategies, and Synergies for Precision Oncology
- PMID: 40564985
- PMCID: PMC12193433
- DOI: 10.3390/ijms26125521
Oleocanthal as a Multifunctional Anti-Cancer Agent: Mechanistic Insights, Advanced Delivery Strategies, and Synergies for Precision Oncology
Abstract
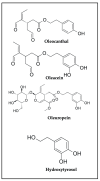
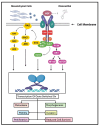
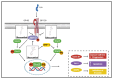
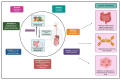
Oleocanthal (OC), a secoiridoid phenolic compound exclusive to extra virgin olive oil (EVOO), has emerged as a promising nutraceutical with multifaceted anti-cancer properties. Despite its well-characterized anti-inflammatory and antioxidant effects, the mechanistic breadth and translational potential of OC in oncology remain underexplored and fragmented across the literature. This comprehensive review synthesizes and critically analyzes recent advances in the molecular, pharmacological, and translational landscape of OC's anti-cancer activities, providing an integrative framework to bridge preclinical evidence with future clinical application. We delineate the pleiotropic mechanisms by which OC modulates cancer hallmarks, including lysosomal membrane permeabilization (LMP)-mediated apoptosis, the inhibition of key oncogenic signaling pathways (c-MET/STAT3, PAR-2/TNF-α, COX-2/mPGES-1), the suppression of epithelial-to-mesenchymal transition (EMT), angiogenesis, and metabolic reprogramming. Furthermore, this review uniquely highlights the emerging role of OC in modulating drug resistance mechanisms by downregulating efflux transporters and sensitizing tumors to chemotherapy, targeted therapies, and immunotherapies. We also examine OC's bidirectional interaction with gut microbiota, underscoring its systemic immunometabolic effects. A major unmet need addressed by this review is the lack of consolidated knowledge regarding OC's pharmacokinetic limitations and drug-drug interaction potential in the context of polypharmacy in oncology. We provide an in-depth analysis of OC's poor bioavailability, extensive first-pass metabolism, and pharmacogenomic interactions, and systematically compile preclinical evidence on advanced delivery platforms-including nanocarriers, microneedle systems, and peptide-drug conjugates-designed to overcome these barriers. By critically evaluating the mechanistic, pharmacological, and translational dimensions of OC, this review advances the field beyond isolated mechanistic studies and offers a strategic blueprint for its integration into precision oncology. It also identifies key research gaps and outlines the future directions necessary to transition OC from a nutraceutical of dietary interest to a viable adjunctive therapeutic agent in cancer treatment.
Keywords: Mediterranean diet; cancer metabolism; gut microbiome; lysosomal membrane permeabilization; nanoparticle drug delivery; oleocanthal; polyphenols; precision oncology.
Conflict of interest statement
The authors declare that they have no competing financial interests or personal relationships which could have influenced the work reported in this paper.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

