Subcortical Circuits Among Pedunculopontine Nucleus, Thalamus and Basal Ganglia Play Important Roles in Paroxysmal Arousal in Genetic Rat Models of Autosomal Dominant Sleep-Related Hypermotor Epilepsy
- PMID: 40564986
- PMCID: PMC12193656
- DOI: 10.3390/ijms26125522
Subcortical Circuits Among Pedunculopontine Nucleus, Thalamus and Basal Ganglia Play Important Roles in Paroxysmal Arousal in Genetic Rat Models of Autosomal Dominant Sleep-Related Hypermotor Epilepsy
Abstract
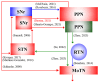
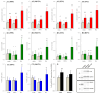
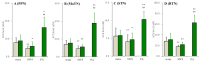
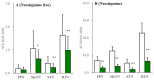
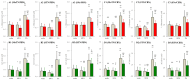
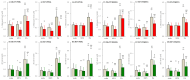
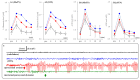
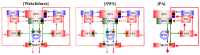
A part of autosomal dominant sleep-related hypermotor epilepsy (ADSHE) is caused by mutant CHRNA4. The pathomechanisms underlying motor seizures followingly brief/sudden awakening (paroxysmal arousal) in ADSHE seizures remain to be clarified. This study determined extracellular levels of ACh and L-glutamate in the pedunculopontine nucleus (PPN) and its projection regions, including the thalamus and basal ganglia, during wakefulness, slow-wave sleep (SWS) and paroxysmal arousal of transgenic rats bearing rat S286L-mutant Chrna4 (S286L-TG), corresponding to human S284L-mutant CHRNA4, using microdialysis. The expression of connexin43 and pannexin1 in the plasma membrane of the PPN was determined using capillary immunoblotting. The expressions of connexin43 and pannexin1 in the PPN plasma membrane of S286L-TG were larger than the wild type. The extracellular L-glutamate levels in the PPN and projection regions of S286L-TG consistently increased during both wakefulness and SWS compared to the wild type. The extracellular levels of ACh and L-glutamate in the PPN and projection regions decreased accompaning SWS in the wild type. In S286L-TG, this decreasing extracellular ACh level was observed, whereas decreasing L-glutamate level was impaired. Both extracellular levels of ACh and L-glutamate in the PPN and projection regions drastically increased during paroxysmal arousal. Hemichannel inhibitors suppressed the increasing releases of ACh and L-glutamate induced by paroxysmal arousal but decreased and did not affect extracellular levels of L-glutamate and ACh during wakefulness and SWS, respectively. In particular, under hemichannels inhibition, decreasing L-glutamate release accompanying SWS was observed in S286L-TG. This study elucidated that enhanced hemichannels are predominantly involved in the dysfunction of glutamatergic transmission compared to AChergic transmission during the interictal stage in S286L-TG, whereas the hyperactivation of hemichannels contributes to the generation of paroxysmal arousal. Therefore, the hyperactivated excitatory tripartite synaptic transmission associated with hemichannels in the PPN and projection regions plays important roles in epileptogenesis/ictogenesis in S286L-TG.
Keywords: ACh; ADSHE; glutamate; hemichannel; tripartite synaptic transmission.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Age-Dependent and Sleep/Seizure-Induced Pathomechanisms of Autosomal Dominant Sleep-Related Hypermotor Epilepsy.Int J Mol Sci. 2020 Oct 30;21(21):8142. doi: 10.3390/ijms21218142. Int J Mol Sci. 2020. PMID: 33143372 Free PMC article.
-
Pathogenesis and pathophysiology of autosomal dominant sleep-related hypermotor epilepsy with S284L-mutant α4 subunit of nicotinic ACh receptor.Br J Pharmacol. 2020 May;177(9):2143-2162. doi: 10.1111/bph.14974. Epub 2020 Feb 15. Br J Pharmacol. 2020. PMID: 31901135 Free PMC article.
-
Age-Dependent Activation of Pannexin1 Function Contributes to the Development of Epileptogenesis in Autosomal Dominant Sleep-related Hypermotor Epilepsy Model Rats.Int J Mol Sci. 2024 Jan 28;25(3):1619. doi: 10.3390/ijms25031619. Int J Mol Sci. 2024. PMID: 38338895 Free PMC article.
-
Association of sleep with sudden unexpected death in epilepsy.Epilepsy Behav. 2017 Nov;76:1-6. doi: 10.1016/j.yebeh.2017.08.021. Epub 2017 Sep 13. Epilepsy Behav. 2017. PMID: 28917499
-
Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection.Cochrane Database Syst Rev. 2011 Jul 6;(7):CD003510. doi: 10.1002/14651858.CD003510.pub3. Cochrane Database Syst Rev. 2011. PMID: 21735394
References
-
- Steinlein O.K., Mulley J.C., Propping P., Wallace R.H., Phillips H.A., Sutherland G.R., Scheffer I.E., Berkovic S.F. A missense mutation in the neuronal nicotinic acetylcholine receptor alpha 4 subunit is associated with autosomal dominant nocturnal frontal lobe epilepsy. Nat. Genet. 1995;11:201–203. doi: 10.1038/ng1095-201. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

