Reduced Gene Dosage of the Psychiatric Risk Gene Cacna1c Is Associated with Impairments in Hypothalamic-Pituitary-Adrenal Axis Activity in Rats
- PMID: 40565009
- PMCID: PMC12192671
- DOI: 10.3390/ijms26125547
Reduced Gene Dosage of the Psychiatric Risk Gene Cacna1c Is Associated with Impairments in Hypothalamic-Pituitary-Adrenal Axis Activity in Rats
Abstract
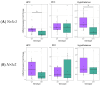
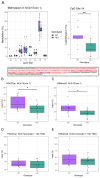
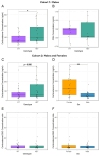
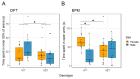
Common and rare variation in CACNA1C gene expression has been consistently associated with neuropsychiatric disorders such as schizophrenia, bipolar disorder, and major depression. However, the underlying biological pathways that cause this association have yet to be fully determined. In this study, we present evidence that rats with a reduced gene dosage of Cacna1c have increased basal corticosterone levels in the periphery and reduced the expression of Nr3c1 encoding the glucocorticoid receptor in the hippocampus and hypothalamus. These results are consistent, with an effect of Cacna1c dosage on hypothalamus-pituitary-adrenal (HPA) axis function. Heterozygous Cacna1c rats had lower levels of the histone markers H3K4me3 and H3K27acat exon 17 of the Nr3c1 gene. These histone modifications are typically linked to increased gene expression, but here were not associated with changes in the expression of exon 17 variants under non-stress conditions. Heterozygous Cacna1c rats additionally show increased anxiety behaviours. These results support an association of Cacna1c heterozygosity with the altered activity of the HPA axis and function in the resting state, and this may be a predisposing mechanism that contributes to the increased risk of psychiatric disorders with stress.
Keywords: Cacna1c; L-type voltage-gated calcium channel; anxiety; bipolar disorder; glucocorticoid; hippocampus; schizophrenia; stress.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Hypothalamic-pituitary-adrenal (HPA) axis suppression after treatment with glucocorticoid therapy for childhood acute lymphoblastic leukaemia.Cochrane Database Syst Rev. 2017 Nov 6;11(11):CD008727. doi: 10.1002/14651858.CD008727.pub4. Cochrane Database Syst Rev. 2017. PMID: 29106702 Free PMC article.
-
Atypical depression and non-atypical depression: Is HPA axis function a biomarker? A systematic review.J Affect Disord. 2018 Jun;233:45-67. doi: 10.1016/j.jad.2017.09.052. Epub 2017 Oct 6. J Affect Disord. 2018. PMID: 29150144
-
Hypothalamic-pituitary-adrenal (HPA) axis suppression after treatment with glucocorticoid therapy for childhood acute lymphoblastic leukaemia.Cochrane Database Syst Rev. 2012 May 16;(5):CD008727. doi: 10.1002/14651858.CD008727.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2015 Aug 17;(8):CD008727. doi: 10.1002/14651858.CD008727.pub3. PMID: 22592733 Updated.
-
How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans.Neurobiol Dis. 2013 Apr;52:24-37. doi: 10.1016/j.nbd.2012.03.012. Epub 2012 Mar 9. Neurobiol Dis. 2013. PMID: 22426398
-
Child maltreatment and NR3C1 exon 1F methylation, link with deregulated hypothalamus-pituitary-adrenal axis and psychopathology: A systematic review.Child Abuse Negl. 2021 Dec;122:105304. doi: 10.1016/j.chiabu.2021.105304. Epub 2021 Sep 3. Child Abuse Negl. 2021. PMID: 34488052
References
-
- Ferreira M.A.R., O’Donovan M.C., Meng Y.A., Jones I.R., Ruderfer D.M., Jones L., Fan J., Kirov G., Perlis R.H., Green E.K., et al. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat. Genet. 2008;40:1056–1058. doi: 10.1038/ng.209. - DOI - PMC - PubMed
-
- Green E.K., Grozeva D., Jones I., Jones L., Kirov G., Caesar S., Gordon-Smith K., Fraser C., Forty L., Russell E., et al. The bipolar disorder risk allele at CACNA1C also confers risk of recurrent major depression and of schizophrenia. Mol. Psychiatry. 2010;15:1016–1022. doi: 10.1038/mp.2009.49. - DOI - PMC - PubMed
-
- Zheng F., Zhang Y., Xie W., Li W., Jin C., Mi W., Wang F., Ma W., Ma C., Yang Y., et al. Further evidence for genetic association of CACNA1C and schizophrenia: New risk loci in a Han Chinese population and a meta-analysis. Schizophr. Res. 2014;152:105–110. doi: 10.1016/j.schres.2013.12.003. - DOI - PubMed
-
- Bigos K.L., Mattay V.S., Callicott J.H., Straub R.E., Vakkalanka R., Kolachana B., Hyde T.M., Lipska B.K., Kleinman J.E., Weinberger D.R. Genetic Variation in CACNA1C Affects Brain Circuitries Related to Mental Illness. Arch. Gen. Psychiatry. 2010;67:939–945. doi: 10.1001/archgenpsychiatry.2010.96. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

