Radiation Promotes Acute and Chronic Damage to Adipose Tissue
- PMID: 40565100
- PMCID: PMC12193394
- DOI: 10.3390/ijms26125626
Radiation Promotes Acute and Chronic Damage to Adipose Tissue
Abstract
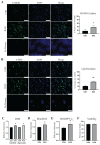
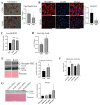
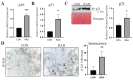
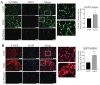
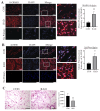
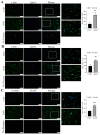
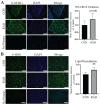
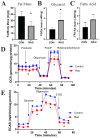
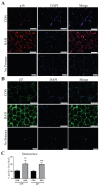
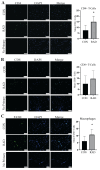
Radiotherapy is commonly used for treating various types of cancer. In addition, adipose tissue is not routinely spared during typical radiation treatment. Although radiation is known to induce metabolic effects in patients, the effects of radiation therapy on adipose tissue have not been elucidated. Currently, few studies have investigated the impact of radiation exposure on adipose tissue, and these have primarily involved whole-body irradiation. This study aimed to understand the acutely persistent damage caused by clinically relevant radiation doses in adipocytes. Specifically, in vitro and in vivo, irradiated adipocytes increased reactive oxygen species (ROS) and lipid peroxidation levels and elevated lipolytic activity compared to unirradiated adipocytes. RNA sequencing also revealed the upregulation of senescence and inflammation pathways. We observed an increase in macrophage and T-cell accumulation at both 1 and 6 months after radiation exposure using in vivo models. Many of the changes observed in irradiated adipose tissue, including oxidative stress, metabolic dysfunction, inflammation, and senescence, are consistent with those observed in adipose tissue from obese patients, in which obesity is a known driver of many cancers. As adipose tissue damage is maintained chronically, protecting adipose tissue from the harmful effects of radiation exposure may improve radiation-induced toxicity and reduce cancer recurrence and progression.
Keywords: adipose tissue; inflammation; metabolism; oxidative stress; radiation; senescence.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
High-fat diet-induced adipose tissue-resident macrophages, T cells, and dendritic cells modulate chronic inflammation and adipogenesis during obesity.Front Immunol. 2025 Jun 3;16:1524544. doi: 10.3389/fimmu.2025.1524544. eCollection 2025. Front Immunol. 2025. PMID: 40529363 Free PMC article.
-
Therapeutic effects of PDGF-AB/BB against cellular senescence in human intervertebral disc.Elife. 2025 Jul 16;13:RP103073. doi: 10.7554/eLife.103073. Elife. 2025. PMID: 40668091 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Adipose tissue protein kinase D: regulation of signaling networks and its sex-dependent effects on metabolism.Am J Physiol Endocrinol Metab. 2025 Jul 1;329(1):E67-E85. doi: 10.1152/ajpendo.00391.2024. Epub 2025 Jun 2. Am J Physiol Endocrinol Metab. 2025. PMID: 40454664
Cited by
-
From mitochondrial dysregulation to ferroptosis: Exploring new strategies and challenges in radioimmunotherapy (Review).Int J Oncol. 2025 Sep;67(3):76. doi: 10.3892/ijo.2025.5781. Epub 2025 Aug 8. Int J Oncol. 2025. PMID: 40776761 Free PMC article. Review.
References
-
- Duval C., Thissen U., Keshtkar S., Accart B., Stienstra R., Boekschoten M.V., Roskams T., Kersten S., Muller M. Adipose tissue dysfunction signals progression of hepatic steatosis towards nonalcoholic steatohepatitis in C57BL/6 mice. Diabetes. 2010;59:3181–3191. doi: 10.2337/db10-0224. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

