In Vitro Correction of Point Mutations in the DYSF Gene Using Prime Editing
- PMID: 40565111
- PMCID: PMC12193300
- DOI: 10.3390/ijms26125647
In Vitro Correction of Point Mutations in the DYSF Gene Using Prime Editing
Abstract
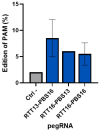
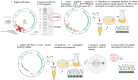
Dysferlinopathy is caused by over 500 mutations in the gene encoding dysferlin, including close to 300 point mutations. One option to cure the disease is to use a gene therapy to correct these mutations at the root. Prime editing is a technique which can replace the mutated nucleotide with the wild-type nucleotide. In this article, prime editing is used to correct several point mutations in the DYSF gene responsible for dysferlinopathy. In vitro editing of HEK293T cells reaches up to 31%. Notably, editing was more efficient in myoblasts than in patient-derived fibroblasts. The prime editing technique was also used to create a new myoblast clone containing a patient mutation from a healthy myoblast cell line.
Keywords: CRISPR; LGMD; Miyoshi Myopathy; dysferlin; dysferlinopathy; gene therapy; point mutation; prime editing.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Straub V., Murphy A., Udd B., Corrado A., Aymé S., Bönneman C., de Visser M., Hamosh A., Jacobs L., Khizanishvili N. 229th ENMC international workshop: Limb girdle muscular dystrophies-Nomenclature and reformed classification Naarden, the Netherlands, 17–19 March 2017. Neuromuscul. Disord. 2018;28:702–710. doi: 10.1016/j.nmd.2018.05.007. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

