Evaluation of the Effects of the Sodium-Glucose Cotransporter 2 Inhibitors and Sacubitril/Valsartan Combined Therapy in Patients with HFrEF: An Echocardiographic Study
- PMID: 40565116
- PMCID: PMC12193552
- DOI: 10.3390/ijms26125651
Evaluation of the Effects of the Sodium-Glucose Cotransporter 2 Inhibitors and Sacubitril/Valsartan Combined Therapy in Patients with HFrEF: An Echocardiographic Study
Abstract
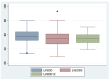
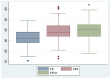
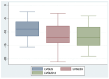
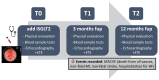
Sodium-glucose cotransporter 2 inhibitors (iSGLT2) have become the fourth pillar of the medical treatment for heart failure with reduced ejection fraction (HFrEF). However, the mechanisms of action of iSGLT2 remain poorly understood. The effectiveness of combined ARNI and iSGLT2 therapy in left ventricular (LV) remodeling is still under study. We aim to investigate the effects of ARNI + iSGLT2 combination therapy in patients affected by HFrEF in terms of ventricular remodeling using speckle tracking echocardiography (STE). In this observational study, 136 patients with HFrEF taking ARNI were enrolled. All patients were evaluated at baseline (before iSGLT2), at 3 months and at 12 months from the beginning of iSGLT2 therapy. Echocardiographic parameters, including STE analysis and volumetric and LV contractile function indices, were collected at the three timepoints. The objectives were (1) to evaluate the effects of ARNI + iSGLT2 combination therapy on ultrasound (US) measurements; (2) to evaluate the effects on the variation of laboratory data indicative of HF (NT-pro-BNP); and (3) to evaluate the medium-long term impact of the ARNI + iSGLT2 combination therapy in terms of major cardiovascular events (MACVE). After only three months of combined ARNI + iSGLT2 therapy, we reported a significant improvement in ventricular and atrial volumetric indices, systolic function indices and myocardial deformation parameters assessed by STE. We also reported a significant decrease in NTproBNP levels. This trend was confirmed at 12 months follow-up. Furthermore, narrowing down the analysis to patients who were already treated with ARNI when they started taking iSGLT2, we reported similar results in the improvement of US parameters and NTproBNP levels. Our study has shown that the ARNI + iSGLT2 combination therapy leads to a clinical improvement and positive ventricular remodeling. Even the single introduction of additional iSGLT-2 in HFrEF patients on an otherwise optimized therapy resulted in a significant improvement in US and laboratory variables. The results of our study suggest implementing iSGLT-2 therapy as soon as possible, as the structural and functional cardiac improvements achieved by these drugs are achieved in the short term and maintained in the long term.
Keywords: echocardiography; gliflozins; global longitudinal strain; heart failure.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

