Magnesium Balance in Chronic Kidney Disease: Mineral Metabolism, Immunosuppressive Therapies and Sodium-Glucose Cotransporter 2 Inhibitors
- PMID: 40565121
- PMCID: PMC12193085
- DOI: 10.3390/ijms26125657
Magnesium Balance in Chronic Kidney Disease: Mineral Metabolism, Immunosuppressive Therapies and Sodium-Glucose Cotransporter 2 Inhibitors
Abstract
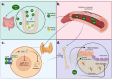
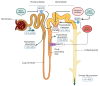
It is now widely recognized that maintaining magnesium (Mg) homeostasis is critical for health, especially in the context of chronic kidney disease (CKD). Patients with CKD commonly develop hyperphosphatemia and secondary hyperparathyroidism, which are controlled by therapies targeting intestinal phosphate absorption and circulating calcium levels or by modulating parathyroid calcium sensing. Notably, Mg supplementation may provide dual benefits by promoting bone formation and maintaining normal mineralization with slightly elevated serum levels. Importantly, low Mg levels are associated with mortality risk in CKD, highlighting the importance of maintaining adequate serum Mg levels in these patients. Particularly, kidney transplant (KT) patients have lower circulating Mg levels, likely due to interactions with immunosuppressive treatments. Sodium-glucose co-transporter 2 (SGLT2) inhibitors have shown survival benefits in CKD and increased serum Mg levels, suggesting that Mg regulation may contribute to these outcomes. Overall, Mg plays a key role in CKD-associated mineral and bone disorders (CKD-MBD). Thus, understanding the mechanisms underlying the alteration of Mg homeostasis in CKD could improve clinical outcomes. This review summarizes the basic and clinical studies demonstrating (1) the key actions of Mg in CKD-MBD, including secondary hyperparathyroidism and bone abnormalities; (2) the distinctive profile of KT patients for Mg homeostasis; and (3) the interaction between commonly used drugs, such as SGLT2 inhibitors or immunosuppressive treatments, and Mg metabolism, providing a broad understanding of both the key role of Mg in the context of CKD and the treatments that should be considered to manage Mg levels in CKD patients.
Keywords: CKD-MBD; SLGT2 inhibitors; chronic kidney disease; magnesium; renal transplant patients.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Insulin and glucose-lowering agents for treating people with diabetes and chronic kidney disease.Cochrane Database Syst Rev. 2018 Sep 24;9(9):CD011798. doi: 10.1002/14651858.CD011798.pub2. Cochrane Database Syst Rev. 2018. PMID: 30246878 Free PMC article.
-
Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers for adults with early (stage 1 to 3) non-diabetic chronic kidney disease.Cochrane Database Syst Rev. 2023 Jul 19;7(7):CD007751. doi: 10.1002/14651858.CD007751.pub3. Cochrane Database Syst Rev. 2023. PMID: 37466151 Free PMC article.
-
Cardiorenal Benefits of SGLT2 Inhibitors in Patients with Chronic Kidney Disease and Concomitant Hypertension.Cardiorenal Med. 2025;15(1):496-509. doi: 10.1159/000545622. Epub 2025 Apr 21. Cardiorenal Med. 2025. PMID: 40258345 Review.
-
Design considerations for future renoprotection trials in the era of multiple therapies for chronic kidney disease.Nephrol Dial Transplant. 2025 Feb 5;40(Supplement_1):i70-i79. doi: 10.1093/ndt/gfae210. Nephrol Dial Transplant. 2025. PMID: 39907541 Free PMC article.
-
Synbiotics, prebiotics and probiotics for people with chronic kidney disease.Cochrane Database Syst Rev. 2023 Oct 23;10(10):CD013631. doi: 10.1002/14651858.CD013631.pub2. Cochrane Database Syst Rev. 2023. PMID: 37870148 Free PMC article.
References
-
- Zhang C., Zhang T., Zou J., Miller C.L., Gorkhali R., Yang J.-Y., Schilmiller A., Wang S., Huang K., Brown E.M., et al. Structural Basis for Regulation of Human Calcium-Sensing Receptor by Magnesium Ions and an Unexpected Tryptophan Derivative Co-Agonist. Sci. Adv. 2016;2:e1600241. doi: 10.1126/sciadv.1600241. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

