Anti-lymphoma Activity of Acyclic Terpenoids and Its Structure-Activity Relationship: In Vivo, In Vitro, and In Silico Studies
- PMID: 40565145
- PMCID: PMC12192932
- DOI: 10.3390/ijms26125683
Anti-lymphoma Activity of Acyclic Terpenoids and Its Structure-Activity Relationship: In Vivo, In Vitro, and In Silico Studies
Abstract
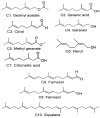
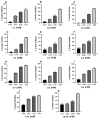
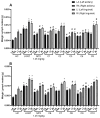
Terpenoids are a large group of molecules present in several plant species and in many essential oils reported with cytotoxic and anticancer properties. The aim of this study was to evaluate the anticancer activity of eleven acyclic terpenes; seven monoterpenoids: geranyl acetate (C1), geranic acid (C2), citral (C3, mixture of neral and geranial), geraniol (C4), methyl geranate (C5), nerol (C6) and citronellic acid (C7); two sesquiterpenes: farnesal (C8) and farnesol (C9); and one triterpene: squalene (C10), using in vivo, in vitro, and in silico models. Anti-lymphoma activity was evaluated using male Balb/c mice inoculated with U-937 cells. Cytotoxic activity was evaluated using the WST-1 method. Computer tools were used to obtain a molecular docking study, measuring pharmacokinetic and toxicological properties of the acyclic terpenoids with greater antitumor activity. The results showed that the terpenoids with the highest cytotoxic and nodal growth inhibitory activity were C3, C4, C6, and C9, and their effects were better compared to MTX. The data obtained suggest that the anti-lymphoma activity could be due to the presence of the aldehyde, hydroxyl, and acetate groups in the C1 of the monoterpenes and sesquiterpenes evaluated. The theoretical results obtained from molecular docking showed that geranial (C3A), neral (C3B), C9, and C6 terpenoids obtained a higher affinity for the HMG-CoA reductase enzyme and suggest that it could be a target to induce anti-lymphoma activity of bioactive terpenoids. Our study provides evidence that C3, C6, and C9 could be potential anticancer agents for the treatment of histiocytic lymphoma.
Keywords: Bcl-2; DHFR; FASN; HMG-CoA reductase; U-937 cells; acyclic terpenoids; anti-lymphoma activity; molecular docking.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Sapkota S., Shaikh H. Non-Hodgkin Lymphoma. StatPearls Publishing; Miami, FL, USA: 2023. StatPearls [Internet] - PubMed
-
- Secretaria de Salud Publica. [(accessed on 18 December 2024)]. Available online: https://www.gob.mx/salud/prensa/294-mexico-registra-al-ano-mas-de-195-mi....
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

