Phenolic Profiling and Bioactive Properties of Arthrospira platensis Extract in Alleviating Acute and Sub-Chronic Colitis
- PMID: 40565157
- PMCID: PMC12193454
- DOI: 10.3390/ijms26125692
Phenolic Profiling and Bioactive Properties of Arthrospira platensis Extract in Alleviating Acute and Sub-Chronic Colitis
Abstract
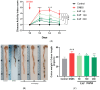
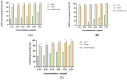
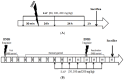
Arthrospira platensis, a filamentous photosynthetic cyanobacterium, is widely recognized for its high nutritional value, broad spectrum of bioactive compounds, and excellent safety profile, making it a promising natural source for health-promoting applications. This study aimed to profile the phenolic constituents of an ethanolic extract of A. platensis (EAP) using HPLC-DAD-ESI-MS and to investigate its pharmacological effects in attenuating acute and sub-chronic experimental colitis, as well as its antioxidant and antifungal properties. Colitis was induced in BALB/c mice by intrarectal administration of 2,4-dinitrobenzenesulfonic acid (DNBS), followed by oral administration of EAP at doses of 50, 100, and 200 mg/kg. Phenolic profiling revealed eight major compounds, with a cumulative content of 6.777 mg/g of extract, with Pyrogallol, Ferulic acid, and Chlorogenic acid being the most abundant. In vivo, EAP treatment significantly reduced the Disease Activity Index (DAI), alleviated macroscopic colonic damage, and preserved colonic mucosal integrity in both inflammatory phases. Biochemical analyses revealed significant reductions in myeloperoxidase (MPO) activity, nitric oxide (NO), and malondialdehyde (MDA) levels, accompanied by increased reduced glutathione (GSH) content and catalase activity. In vitro, EAP demonstrated notable antioxidant effects, including 56% DPPH and 47% ABTS radical scavenging activities, and an 81% ferrous ion-chelating capacity. Furthermore, it exhibited antifungal activity, with inhibition zones of 20 mm against Candida albicans and 15 mm against Aspergillus flavus, respectively. These findings highlight the multitarget bioactivity of EAP and support its potential as a natural agent for managing intestinal inflammation and oxidative stress across both acute and sub-chronic phases.
Keywords: DNBS-induced colitis; HPLC-DAD-ESI-MS; phenolic compounds; spirulina.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



 ) submucosal edema; (
) submucosal edema; ( ) infiltration of mononuclear cells in the submucosa; (
) infiltration of mononuclear cells in the submucosa; ( ) disruption of crypt integrity; (
) disruption of crypt integrity; ( ) necrotic areas. M: mucosa. SM: submucosa. ME: muscularis externa.
) necrotic areas. M: mucosa. SM: submucosa. ME: muscularis externa.
 ) submucosal edema; (
) submucosal edema; ( ) infiltration of mononuclear cells in the submucosa; (
) infiltration of mononuclear cells in the submucosa; ( ) disruption of crypt integrity; (
) disruption of crypt integrity; ( ) necrotic areas. M: mucosa. SM: submucosa. ME: muscularis externa.
) necrotic areas. M: mucosa. SM: submucosa. ME: muscularis externa.

 ) submucosal edema; (
) submucosal edema; ( ) infiltration of mononuclear cells in the submucosa; (
) infiltration of mononuclear cells in the submucosa; ( ) disruption of crypt integrity; (
) disruption of crypt integrity; ( ) granulomas. M: mucosa. SM: submucosa. ME: muscularis externa.
) granulomas. M: mucosa. SM: submucosa. ME: muscularis externa.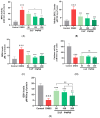
References
-
- McDowell C., Farooq U., Haseeb M. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2022. Inflammatory Bowel Disease. - PubMed
-
- Bribi N., Rodríguez-Nogales A., Vezza T., Algieri F., Rodriguez-Cabezas M.E., Garrido-Mesa J., Gálvez J. Intestinal Anti-Inflammatory Activity of the Total Alkaloid Fraction from Fumaria capreolata in the DSS Model of Colitis in Mice. Bioorg. Med. Chem. Lett. 2020;30:127414. doi: 10.1016/j.bmcl.2020.127414. - DOI - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

