Targeting WEE1 Kinase for Breast Cancer Therapeutics: An Update
- PMID: 40565165
- PMCID: PMC12192920
- DOI: 10.3390/ijms26125701
Targeting WEE1 Kinase for Breast Cancer Therapeutics: An Update
Abstract
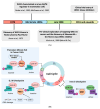
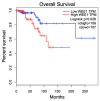
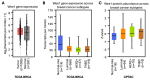
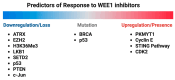
WEE1 kinase is a crucial cell cycle regulatory protein that controls the timing of mitotic entry. WEE1, via inhibition of Cyclin-dependent Kinase 1 (CDK1) and Cyclin-dependent Kinase 2 (CDK2), governs the G2-M checkpoint by inhibiting entry into mitosis. The state of balance between WEE family kinases and CDC25C phosphatases restricts CDK1/CycB activity. The WEE kinase family consists of WEE1, PKMYT1, and WEE2 (WEE1B). WEE1 and PKMYT1 regulate entry into mitosis during cell cycle progression, whereas WEE2 governs cell cycle progression during meiosis. Recent studies have identified WEE1 as a potential therapeutic target in several cancers, including therapy-resistant triple-negative breast cancer. Adavosertib's clinical promise was challenged by inter-individual variations in response and side effects. Because of these promising preclinical outcomes, other WEE1 kinase inhibitors (Azenosertib, SC0191, IMP7068, PD0407824, PD0166285, WEE1-IN-5, Zedoresertib, WEE1-IN-8, and ATRN-1051) are being developed, with several currently being evaluated in clinical trials or as an adjuvant to chemotherapies. Preclinical studies show WEE1 inhibitors induce MHC class 1 antigens and STING when given as combination therapies, suggesting potential additional therapeutic opportunities. Reliable predictors of clinical responses based on mechanistic insights remain an important unmet need. Herein, we review the role of WEE1 inhibition therapy in breast cancer.
Keywords: DNA damage/repair; G2/M checkpoint; WEE1 inhibitors; WEE1 kinase; breast cancer; cell cycle regulation; chemotherapy resistance; clinical trial; cyclin-dependent kinases; mitotic entry.
Conflict of interest statement
R.G.P. holds ownership interests in CytoDyn, LightSeed, StromaGenesis. ioROC, Shenandoah, and EcoGenome, and several patents and submitted patent applications. R.G.P. is a consultant for CytoDyn.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

