Bacteria Under Metal Stress-Molecular Mechanisms of Metal Tolerance
- PMID: 40565180
- PMCID: PMC12193353
- DOI: 10.3390/ijms26125716
Bacteria Under Metal Stress-Molecular Mechanisms of Metal Tolerance
Abstract
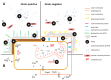
Metals are natural components of the lithosphere, whose amounts and bioavailability are increasing in many areas due to their continuous release from both natural sources and intensive human activities. Some metals are essential or beneficial for living organisms, while others are non-essential and potentially toxic. When present at higher concentrations, even essential and beneficial metal ions can become harmful to all forms of life. Bacteria, unicellular organisms that have been exposed to metals since the earliest stages of life on Earth, have evolved metabolic pathways involving essential metals as well as diverse strategies to cope with metal toxicity. In the domain Bacteria, two main strategies have been identified: (i) metal exclusion, which includes cell wall sequestration and immobilization of metals in extracellular exopolysaccharides, siderophores, and other soluble microbial products, as well as (ii) metal tolerance, involving intracellular sequestration of metals (e.g., by metallothioneins, or low molecular weight thiols) as well as enzymatic conversion of metals to less toxic forms and/or its active efflux. Microorganisms possessing such adaptive traits are considered valuable agents for potential application in medicine, environmental sciences, and bioengineering (e.g., bioremediation and/or biomining).
Keywords: enzymatic detoxification; exopolysaccharides; metal efflux; metallothionein; siderophores.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
Measures implemented in the school setting to contain the COVID-19 pandemic.Cochrane Database Syst Rev. 2022 Jan 17;1(1):CD015029. doi: 10.1002/14651858.CD015029. Cochrane Database Syst Rev. 2022. Update in: Cochrane Database Syst Rev. 2024 May 2;5:CD015029. doi: 10.1002/14651858.CD015029.pub2. PMID: 35037252 Free PMC article. Updated.
-
Education support services for improving school engagement and academic performance of children and adolescents with a chronic health condition.Cochrane Database Syst Rev. 2023 Feb 8;2(2):CD011538. doi: 10.1002/14651858.CD011538.pub2. Cochrane Database Syst Rev. 2023. PMID: 36752365 Free PMC article.
-
Psychological interventions for adults who have sexually offended or are at risk of offending.Cochrane Database Syst Rev. 2012 Dec 12;12(12):CD007507. doi: 10.1002/14651858.CD007507.pub2. Cochrane Database Syst Rev. 2012. PMID: 23235646 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Domínguez D.C. Calcium signaling in Procaryotes. In: Buchholz J.N., Behringer E.J., editors. Calcium and Signal Transduction. IntechOpen; London, UK: 2018. pp. 89–106. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

