IL-1b-Bearing NETs: Bridging Inflammation to Early Cirrhosis in Hepatitis B
- PMID: 40565198
- PMCID: PMC12193664
- DOI: 10.3390/ijms26125733
IL-1b-Bearing NETs: Bridging Inflammation to Early Cirrhosis in Hepatitis B
Abstract
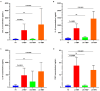
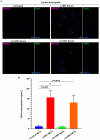
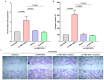
Hepatitis B virus (HBV) infection is one of the most dangerous viral diseases, with innate immunity representing the first line of defense against the virus. In this branch of the immune system, neutrophils are considered key cellular mediators. To better understand the implication of neutrophils in the distinct stages of the disease, HBV-infected patients were enrolled in this study and categorized into three groups: patients with acute infection, chronic infection under treatment, and at early cirrhotic stage. To elucidate the role of inflammatory mediators and cellular mechanisms of neutrophilic origin in the course of the infection, both ex vivo and in vitro studies were performed. Increased levels of C-C motif chemokine ligand 2 (CCL2), interleukin (IL)-18, IL-33, and citrullinated histone H3 (CitH3)-an accurate marker of neutrophil extracellular traps (NETs)-were detected in the circulation of patients with acute infection or early cirrhosis. In parallel, sera from the aforementioned patient groups induced the formation of IL-1b-bearing NETs in neutrophils from healthy individuals. These inflammatory NETs affected primary fibroblasts towards acquiring a pro-fibrotic phenotype. These results suggest that NETs could be regarded as mediators in hepatitis B manifestations, while their therapeutic targeting could enhance the management of early-stage cirrhotic patients.
Keywords: cirrhosis; hepatitis B virus (HBV); inflammation; interleukin (IL)-1b; liver fibrosis; neutrophil extracellular traps; neutrophils.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
IL-1beta expressing neutrophil extracellular traps in Legionella pneumophila infection.Front Immunol. 2025 Jun 6;16:1573151. doi: 10.3389/fimmu.2025.1573151. eCollection 2025. Front Immunol. 2025. PMID: 40547021 Free PMC article.
-
NK-cell-elicited gasdermin-D-dependent hepatocyte pyroptosis induces neutrophil extracellular traps that facilitate HBV-related acute-on-chronic liver failure.Hepatology. 2025 Mar 1;81(3):917-931. doi: 10.1097/HEP.0000000000000868. Epub 2024 Mar 27. Hepatology. 2025. PMID: 38537134
-
NIH Consensus Statement on Management of Hepatitis C: 2002.NIH Consens State Sci Statements. 2002 Jun 10-12;19(3):1-46. NIH Consens State Sci Statements. 2002. PMID: 14768714
-
Hepatitis B immunoglobulin during pregnancy for prevention of mother-to-child transmission of hepatitis B virus.Cochrane Database Syst Rev. 2017 Feb 11;2(2):CD008545. doi: 10.1002/14651858.CD008545.pub2. Cochrane Database Syst Rev. 2017. PMID: 28188612 Free PMC article.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.Health Technol Assess. 2006 Aug;10(28):iii-iv, xi-xiv, 1-183. doi: 10.3310/hta10280. Health Technol Assess. 2006. PMID: 16904047
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

