Ferritin in Acute Myeloid Leukemia: Not Only a Marker of Inflammation and Iron Overload, but Also a Regulator of Cellular Iron Metabolism, Signaling and Communication
- PMID: 40565207
- PMCID: PMC12193126
- DOI: 10.3390/ijms26125744
Ferritin in Acute Myeloid Leukemia: Not Only a Marker of Inflammation and Iron Overload, but Also a Regulator of Cellular Iron Metabolism, Signaling and Communication
Abstract
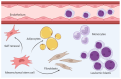
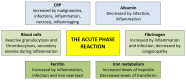
Ferritin is important for cellular iron storage and metabolism. It consists of 24 ferritin heavy- or light-chain subunits surrounding an iron-containing core, but it is also released as an extracellular molecule that shows increased systemic levels during acute-phase reactions. Furthermore, acute myeloid leukemia (AML) is an aggressive bone marrow malignancy that can be associated with increased ferritin levels both at the time of first diagnosis but also during/following anti-AML treatment due to an iron overload. Such high systemic ferritin levels at diagnosis or later allogeneic stem cell transplantation are associated with decreased long-term survival. Extracellular ferritin binds to several receptors expressed by AML cells (e.g., the transferrin receptor and CXCR4 chemokine receptor) and AML-supporting non-leukemic bone marrow cells (e.g., endothelial, mesenchymal or immunocompetent cells). Ferritin can thereby affect the AML cells directly as well as indirectly via AML-supporting neighboring cells. Finally, ferritin should be regarded as a regulator of the dysfunctional iron metabolism that causes increased iron levels in AML cells, and it is important for cell survival through its function during the initial steps of ferroptosis. Thus, ferritin is not only an adverse prognostic biomarker, but also an important regulator of AML cell proliferation, survival and chemosensitivity and the targeting of iron metabolism/ferroptosis is, therefore, a possible strategy in AML therapy.
Keywords: acute myeloid leukemia; acute-phase reaction; chemotherapy; ferritin; ferroptosis; iron; prognosis; stem cell transplantation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Arber D.A., Orazi A., Hasserjian R.P., Borowitz M.J., Calvo K.R., Kvasnicka H.M., Wang S.A., Bagg A., Barbui T., Branford S., et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: Integrating morphologic, clinical, and genomic data. Blood. 2022;140:1200–1228. doi: 10.1182/blood.2022015850. - DOI - PMC - PubMed
-
- Brenner A.K., Tvedt T.H., Nepstad I., Rye K.P., Hagen K.M., Reikvam H., Bruserud Ø. Patients with acute myeloid leukemia can be subclassified based on the constitutive cytokine release of the leukemic cells; the possible clinical relevance and the importance of cellular iron metabolism. Expert Opin. Ther. Targets. 2017;21:357–369. doi: 10.1080/14728222.2017.1300255. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

