Quercetin as a Therapeutic Option in a Rat Model of Aluminum Chloride- and D-Galactose-Induced Neurodegeneration
- PMID: 40565209
- PMCID: PMC12192584
- DOI: 10.3390/ijms26125743
Quercetin as a Therapeutic Option in a Rat Model of Aluminum Chloride- and D-Galactose-Induced Neurodegeneration
Abstract
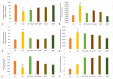
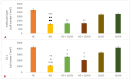
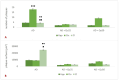
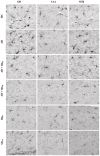
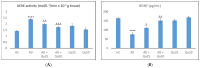
Aluminum (Al) is one of the most abundant metals on Earth and is well known as an environmental neurotoxic agent in the pathogenesis of Alzheimer's disease. Aluminum toxicity is associated with oxidative stress, reduction of antioxidant enzymes, and disruption of the balance of cellular metals, such as iron (Fe), calcium (Ca), and copper (Cu), which causes structural and functional changes in the nervous tissue of the brain or peripheral nervous system. The intake of functional foods, rich in antioxidants, such as quercetin, may be beneficial in combating oxidative stress and neurodegenerative changes in the brain. The aim of this study was to provide deeper insight into the cellular and molecular neuroprotective effects of quercetin in regulating amyloid-beta (Aβ) accumulation, tau pathology, and neuroinflammation in the Al/D-galactose-induced rat model (Al/D-gal) of AD. The results showed that quercetin successfully modulated the impaired homeostatic and neuropathological consequences of aluminum chloride and D-galactose administration over 28 days: it directly protected neurons by regulating the level of oxidative stress and antioxidants, reduced Aβ aggregation by inhibiting the activity of acetylcholinesterase (AChE), increased the survival, growth, and differentiation of nerve cells by maintaining the level of brain-derived neurotrophic factor (BDNF), and regulated microglial immunoreactivity and neuroinflammation by reducing the level of proinflammatory cytokines. The multiple effects confirm that quercetin can be applied as an alternative non-pharmaceutical approach in reducing Al-induced neurotoxicity and maintaining adaptive homeostasis, which consequently affects the functioning of the central nervous system and the whole organism.
Keywords: aluminum/D-galactose-induced Alzheimer’s disease; cellular and molecular neuroprotective effects of quercetin; metal disbalance; molecular insight; neurodegeneration; oxidative-inflammatory markers; quercetin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Empagliflozin and memantine combination ameliorates cognitive impairment in scopolamine + heavy metal mixture-induced Alzheimer's disease in rats: role of AMPK/mTOR, BDNF, BACE-1, neuroinflammation, and oxidative stress.Inflammopharmacology. 2025 Jun;33(6):3479-3498. doi: 10.1007/s10787-025-01755-5. Epub 2025 May 5. Inflammopharmacology. 2025. PMID: 40325262 Free PMC article.
-
Quercetin Ameliorates Learning and Memory in OVX/D-gal-Induced Alzheimer's Disease in Rats by Inhibiting Neuroinflammation via cGAS-STING Signal Pathway.Am J Chin Med. 2025;53(5):1477-1499. doi: 10.1142/S0192415X25500569. Epub 2025 Jul 15. Am J Chin Med. 2025. PMID: 40663436
-
Assessment of the Therapeutic Potential of Hesperidin and Quercetin Nanoparticles on Oxidative Stress, Enzyme Activities, Trophic Factors, and Monoamines in an Animal Model of Depression.Neurochem Res. 2025 Jun 16;50(4):203. doi: 10.1007/s11064-025-04447-2. Neurochem Res. 2025. PMID: 40522360 Free PMC article.
-
Targeting Acetylcholinesterase with Oxytocin: A New Avenue in Alzheimer's Disease Therapeutics.ACS Chem Neurosci. 2025 Jul 2;16(13):2336-2339. doi: 10.1021/acschemneuro.5c00383. Epub 2025 Jun 17. ACS Chem Neurosci. 2025. PMID: 40527057 Review.
-
The Potential Benefits of Quercetin for Brain Health: A Review of Anti-Inflammatory and Neuroprotective Mechanisms.Int J Mol Sci. 2023 Mar 28;24(7):6328. doi: 10.3390/ijms24076328. Int J Mol Sci. 2023. PMID: 37047299 Free PMC article. Review.
Cited by
-
Cyclodextrin-Based Quercetin Powders for Potential Nose-to-Brain Transport: Formulation and In Vitro Assessment.Molecules. 2025 Jul 7;30(13):2878. doi: 10.3390/molecules30132878. Molecules. 2025. PMID: 40649391 Free PMC article.
References
-
- Mahdi O., Baharuldin M.T.H., Mohd Nor N.H., Chiroma S.M., Jagadeesan S., Moklas M.A.M. Chemicals used for the induction of Alzheimer’s disease-like cognitive dysfunctions in rodents. Biomed. Res. Ther. 2019;6:3460–3484. doi: 10.15419/bmrat.v6i11.575. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

