A Study on the Temperature-Dependent Behavior of Small Heat Shock Proteins from Methanogens
- PMID: 40565211
- PMCID: PMC12193508
- DOI: 10.3390/ijms26125748
A Study on the Temperature-Dependent Behavior of Small Heat Shock Proteins from Methanogens
Abstract
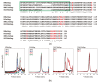
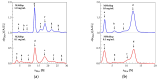
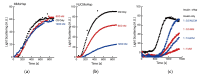
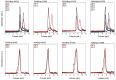
Small heat shock proteins (sHsps) are ubiquitous low-molecular-weight chaperones that prevent protein aggregation under cellular stress conditions. In the absence of stress, they assemble into large oligomers. In response to stress, such as elevated temperatures, they undergo conformational changes that expose hydrophobic surfaces, allowing them to interact with denatured proteins. At heat shock temperatures in bacteria, large sHsp oligomers disassemble into smaller oligomeric forms. Methanogens are a diverse group of microorganisms, ranging from thermophilic to psychrophilic and halophilic species. Accordingly, their sHsps exhibit markedly different temperature dependencies based on their optimal growth temperatures. In this study, we characterized sHsps from both hyperthermophilic and mesophilic methanogens to investigate the mechanisms underlying their temperature-dependent behavior. Using analytical ultracentrifugation, we observed the dissociation of sHsps from a mesophilic methanogen into dimers. The dissociation equilibrium of these oligomers was found to be dependent not only on temperature but also on protein concentration. Furthermore, by generating various mutants, we identified the specific amino acid residues responsible for the temperature dependency observed. The C-terminal region containing the IXI/V motif and the α-crystallin domain were found to be the primary determinants of oligomer stability and its temperature dependence.
Keywords: analytical ultracentrifuge; chaperone; methanogen; small heat shock protein; stress.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Carra S., Alberti S., Arrigo P.A., Benesch J.L., Benjamin I.J., Boelens W., Bartelt-Kirbach B., Brundel B., Buchner J., Bukau B., et al. The growing world of small heat shock proteins: From structure to functions. Cell Stress Chaperones. 2017;22:601–611. doi: 10.1007/s12192-017-0787-8. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

