Molecular Insight into the Recognition of DNA by the DndCDE Complex in DNA Phosphorothioation
- PMID: 40565227
- PMCID: PMC12193691
- DOI: 10.3390/ijms26125765
Molecular Insight into the Recognition of DNA by the DndCDE Complex in DNA Phosphorothioation
Abstract
In a vast variety of prokaryotes such as Escherichia coli and Streptomyces lividans, the DNA degradation (Dnd) CDE protein complex (consisting of DndC, DndD, and DndE), together with the DndA/IscS protein and the DndFGH complex, function as a defense barrier to prevent the invasion of non-self-DNA. The DndCDE complex introduces phosphorothioation (PT) modifications into DNA, and the DndFGH complex specifically cleaves non-PT DNA and, thus, restricts horizontal gene transfer and phage invasion. Despite the central importance of the DndCDE complex in DNA PT modification, which catalyzes the oxygen-sulfur swap on DNA, our understanding of this key complex remains poor. Here, we employed protein structure prediction to provide a reasonably reliable prediction of the structure of the DndCDE complex and a 23 bp DNA-DndCDE complex. We found that among the three proteins in the DndCDE complex, DndC, especially its "specificity loop", plays a key role in recognizing the consensus PT modification sequence. In addition, the DndD protein is found to possess a highly conserved structural surface on its globular domain, presumably mediating the dimerization of DndD as well as the DndCDE complex. Furthermore, our normal mode analysis showed that there exists a dynamic transition between a closed and an open state for the DndCDE complex, facilitating its association and release of DNA. Our conclusions were corroborated by biochemical assays using purified proteins. On the whole, we provide molecular insights into the assembly and DNA-recognition mechanism of a central protein complex involved in DNA phosphorothioation.
Keywords: DNA-DndCDE complex; DndC; DndCDE complex; phosphorothioation modification; specificity loop.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






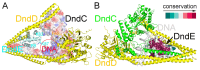

Similar articles
-
Mechanistic insights into JSS1_004-mediated antagonism of the DndBCDE-FGH restriction system and engineering applications.mBio. 2025 Aug 13;16(8):e0138625. doi: 10.1128/mbio.01386-25. Epub 2025 Jul 14. mBio. 2025. PMID: 40657915 Free PMC article.
-
The Crystal Structure and Biochemical Analyses of Escherichia coli YqgF Illuminate Its Diverse Functions.J Mol Biol. 2025 Sep 1;437(17):169221. doi: 10.1016/j.jmb.2025.169221. Epub 2025 May 19. J Mol Biol. 2025. PMID: 40398672
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Phosphorothioated DNA Is Shielded from Oxidative Damage.Appl Environ Microbiol. 2019 Apr 4;85(8):e00104-19. doi: 10.1128/AEM.00104-19. Print 2019 Apr 15. Appl Environ Microbiol. 2019. PMID: 30737351 Free PMC article.
-
Maternal and neonatal outcomes of elective induction of labor.Evid Rep Technol Assess (Full Rep). 2009 Mar;(176):1-257. Evid Rep Technol Assess (Full Rep). 2009. PMID: 19408970 Free PMC article.
References
-
- Ahlgren N.A., Chen Y., Needham D.M., Parada A.E., Sachdeva R., Trinh V., Chen T., Fuhrman J.A. Genome and epigenome of a novel marine Thaumarchaeota strain suggest viral infection, phosphorothioation DNA modification and multiple restriction systems. Environ. Microbiol. 2017;19:2434–2452. doi: 10.1111/1462-2920.13768. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

