Can Nature Overcome Invasive Gastrointestinal Infections?
- PMID: 40565258
- PMCID: PMC12192637
- DOI: 10.3390/ijms26125795
Can Nature Overcome Invasive Gastrointestinal Infections?
Abstract
Invasive bacterial gastrointestinal infections represent a substantial clinical burden worldwide, contributing to significant morbidity and, in severe cases, mortality. The causative bacterial agents of these infections include Shigella spp., enteroinvasive Escherichia coli, Salmonella spp., Campylobacter jejuni, Yersinia enterocolitica, and Listeria monocytogenes. Given the growing challenges of therapy failures and rising antibiotic resistance, there is still an unmet need to identify novel, effective, and safe compounds exhibiting antimicrobial, anti-inflammatory, and immunomodulatory activities. In the present review, we aimed to compile current data regarding three alkaloids-berberine, sanguinarine, and cheleritrin-which hold significant promise in treating bacterial invasive gastrointestinal diseases. Our review extended beyond the direct antimicrobial properties of these compounds against pathogens capable of breaching the intestinal epithelial barrier. We also presented their modulatory effects on intestinal barrier integrity and their influence on the composition and function of the resident gut microbiota, thereby highlighting their potential indirect role in attenuating pathogen invasion and disease progression. Thus, our review presents alkaloids as potential preparations that potentiate the activity of classic anti-infective drugs, as well as substances that, by affecting the microbiome and intestinal mucosa, could be used for inflammatory bowel diseases.
Keywords: berberine; cheleritrin; intestinal barrier; invasion; permeability; sanguinarine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


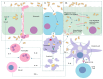


 ) and increases the level of anti-inflammatory cytokines (green square
) and increases the level of anti-inflammatory cytokines (green square  ) [181,187,189]. (C) SAN strengthens the intestinal barrier function through improving the expression of tight junction proteins, especially ZO-1 [186].
) [181,187,189]. (C) SAN strengthens the intestinal barrier function through improving the expression of tight junction proteins, especially ZO-1 [186].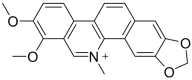
References
-
- [(accessed on 15 March 2025)]. Available online: https://www.who.int/data/gho/data/themes/who-estimates-of-the-global-bur....
-
- Marchello C.S., Birkhold M., Crump J.A., Martin L.B., Ansah M.O., Breghi G., Canals R., Fiorino F., Gordon M.A., Kim J.H., et al. Complications and Mortality of Non-Typhoidal Salmonella Invasive Disease: A Global Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2022;22:692–705. doi: 10.1016/S1473-3099(21)00615-0. - DOI - PMC - PubMed
-
- Berthenet E., Thépault A., Chemaly M., Rivoal K., Ducournau A., Buissonnière A., Bénéjat L., Bessède E., Mégraud F., Sheppard S.K., et al. Source Attribution of Campylobacter Jejuni Shows Variable Importance of Chicken and Ruminants Reservoirs in Non-Invasive and Invasive French Clinical Isolates. Sci. Rep. 2019;9:8098. doi: 10.1038/s41598-019-44454-2. - DOI - PMC - PubMed
-
- Uliczka F., Pisano F., Schaake J., Stolz T., Rohde M., Fruth A., Strauch E., Skurnik M., Batzilla J., Rakin A., et al. Unique Cell Adhesion and Invasion Properties of Yersinia enterocolitica O:3, the Most Frequent Cause of Human Yersiniosis. PLoS Pathog. 2011;7:e1002117. doi: 10.1371/journal.ppat.1002117. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

