Kringle-Dependent Inhibition of Plasmin-Mediated Fibrinolysis by Native and Citrullinated Core Histones
- PMID: 40565263
- PMCID: PMC12193439
- DOI: 10.3390/ijms26125799
Kringle-Dependent Inhibition of Plasmin-Mediated Fibrinolysis by Native and Citrullinated Core Histones
Abstract
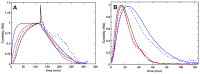
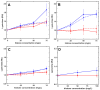
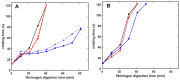
The fibrin matrix of thrombi is intertwined with neutrophil extracellular traps (NETs) containing histones that render resistance to fibrinolysis. During NET formation, histones are citrullinated. Our study addresses the question of whether citrullination modifies the fibrin-stabilizing effects of histones. We studied the structure and viscoelastic properties of fibrin formed in the presence of native or citrullinated H1 and core histones by scanning electron microscopy, clot permeation, and oscillation rheometry. The kinetics of fibrin formation and its dissolution were followed by turbidimetry and thromboelastometry. Co-polymerizing H1 with fibrin enhanced the mechanical strength of the clots, thickened the fibrin fibers, and enlarged the gel pores. In contrast, the addition of core histones resulted in a reduction in the fiber diameter, and the pores were only slightly larger, whereas the mechanical stability was not modified. Plasmin-mediated fibrinogen degradation was delayed by native and citrullinated core histones, but not by H1, and the action of des-kringle1-4-plasmin was not affected. Plasmin-mediated fibrinolysis was inhibited by native and citrullinated core histones, and this effect was moderated when the kringle domains of plasmin were blocked or deleted. These findings suggest that in NET-containing thrombi that are rich in core histones, alternative fibrinolytic enzymes lacking kringle domains are more efficient lytic agents than the classic plasmin-dependent fibrinolysis.
Keywords: fibrin; neutrophil extracellular traps; thrombosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

