Bacillibactin, a Potential Bacillus-Based Antibacterial Non-Ribosomal Peptide: In Silico Studies for Targeting Common Fish Pathogens
- PMID: 40565273
- PMCID: PMC12192977
- DOI: 10.3390/ijms26125811
Bacillibactin, a Potential Bacillus-Based Antibacterial Non-Ribosomal Peptide: In Silico Studies for Targeting Common Fish Pathogens
Abstract
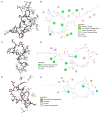
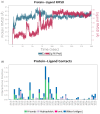
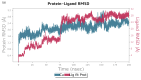
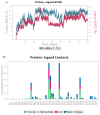
Aquaculture is one of the fastest-growing sectors in food production. The widespread use of antibiotics in fish farming has been identified as a driver for the development of antibiotic resistance. One of the promising approaches to solving this problem is the use of probiotics. There are many promising aquaculture probiotics in the Bacillus genus, which produces non-ribosomal peptides (NRPs). NRPs are known as antimicrobial agents, although evidence is gradually accumulating that they may have other effects, especially at lower (subinhibitory) concentrations. The mechanisms of action of many NRPs remain unexplored, and molecular docking and molecular dynamics studies are invaluable tools for studying such mechanisms. The purpose of this study was to investigate the in silico inhibition of crucial bacterial targets by NRPs. Molecular docking analyses were conducted to assess the binding affinities of the NRPs of Bacillus for protein targets. Among the complexes evaluated, bacillibactin with glutamine synthetase, dihydrofolate reductase, and proaerolysin exhibited the lowest docking scores. Consequently, these complexes were selected for further investigation through molecular dynamics simulations. As a result, three additional potential mechanisms of action for bacillibactin were identified through in silico analyses, including the inhibition of glutamine synthetase, dihydrofolate reductase, and proaerolysin, which are critical bacterial enzymes and considered as the potential antibacterial targets. These findings were further supported by in vitro antagonism assays using bacillibactin-producing Bacillus velezensis strains MT55 and MT155, which demonstrated strong inhibitory activity against Pseudomonas aeruginosa and Aeromonas veronii.
Keywords: aquaculture probiotics; bacillibactin; fengycin; molecular docking; molecular dynamics; surfactin.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of this manuscript; or in the decision to publish the results.
Figures









References
-
- Dimopoulou A., Theologidis I., Benaki D., Koukounia M., Zervakou A., Tzima A., Diallinas G., Hatzinikolaou D.G., Skandalis N. Direct Antibiotic Activity of Bacillibactin Broadens the Biocontrol Range of Bacillus amyloliquefaciens MBI600. mSphere. 2021;6:e00376-21. doi: 10.1128/mSphere.00376-21. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

