A Randomized Controlled Trial Evaluating the Effects of a Probiotic Containing Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 on Gastrointestinal Symptoms and Metabolomic Profiles in Female Dancers
- PMID: 40565286
- PMCID: PMC12193121
- DOI: 10.3390/ijms26125823
A Randomized Controlled Trial Evaluating the Effects of a Probiotic Containing Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 on Gastrointestinal Symptoms and Metabolomic Profiles in Female Dancers
Abstract
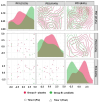
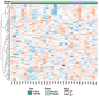
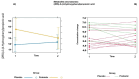
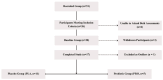
Dancers experience physical and psychological stressors that can impact gut health. We hypothesized that a three-month supplementation with Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 would result in measurable alterations in the fecal metabolomic profile and improve gastrointestinal symptomatology in dancers. Of the 51 volunteers, 26 female dancers were randomized to a 12-week trial (NCT05567653). A homogenous group of 16 (probiotic: n = 5; placebo: n = 11) was analyzed. The participants received L. helveticus R0052 and B. longum R0175 (3 × 109 colony-forming units/day) or a placebo. Baseline dietary intake and body composition were recorded. Fecal samples were analyzed using liquid chromatography-mass spectrometry, and gastrointestinal symptoms were assessed with the Rome IV questionnaire. Statistical methods included principal component analysis, mixed-effects models, and analysis of variance-simultaneous component analysis (ASCA). The study revealed shifts in the probiotic group's fecal metabolome (permutation test p = 0.026), including a reduction in (2RS)-2-(4-hydroxyphenyl)propionic acid (p = 0.023). No improvement in gastrointestinal symptoms was observed. No adverse events occurred. L. helveticus R0052 and B. longum R0175 may alter the gut metabolome, notably (2RS)-2-(4-hydroxyphenyl)propionic acid, but small sample size and absent symptom improvement limit the conclusions. Larger studies with varied doses and blood metabolite analysis are needed to confirm relevance.
Keywords: Bifidobacterium longum R0175; Lactobacillus helveticus R0052; gut metabolome; probiotics; sports nutrition.
Conflict of interest statement
Igor Łoniewski is a probiotic company shareholder, Mariusz Kaczmarczyk and Daniel Styburski are probiotic company employees, and Karolina Skonieczna-Żydecka receives renumeration from a probiotic company. However, this played no role in data presentation and publication. The results of this study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

