Dysregulation of Inositol Polyphosphate 5-Phosphatase OCRL in Alzheimer's Disease: Implications for Autophagy Dysfunction
- PMID: 40565289
- PMCID: PMC12192959
- DOI: 10.3390/ijms26125827
Dysregulation of Inositol Polyphosphate 5-Phosphatase OCRL in Alzheimer's Disease: Implications for Autophagy Dysfunction
Abstract
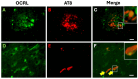
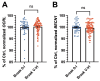
Autophagy is impaired in Alzheimer's disease (AD), particularly at the stage of autophagosome-lysosome fusion. Recent studies suggest that the inositol polyphosphate 5-phosphatase OCRL (Lowe oculocerebrorenal syndrome protein) is involved in this fusion process; however, its role in AD pathophysiology remains largely unclear. In this study, we investigated the localization and expression of OCRL in post-mortem AD brains and in a 5XFAD transgenic mouse model. While OCRL RNA levels were not significantly altered, OCRL protein was markedly reduced in the RIPA-soluble fraction and positively correlated with the autophagy marker Beclin1. Immunohistochemical analysis revealed OCRL immunoreactivity in neuronal cytoplasm, granulovacuolar degeneration bodies, and plaque-associated dystrophic neurites in AD brains. Furthermore, OCRL overexpression in a FRET-based tau biosensor cell model significantly reduced the tau-seeding-induced FRET signal. These findings suggest that OCRL dysregulation may contribute to autophagic deficits and the progression of tau pathology in AD.
Keywords: Alzheimer’s disease; Beclin1; OCRL; amyloid ß; autophagy; pTau; phosphatidylinositol; tau.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Brion J.P., Couck A.M., Passareiro E., Flament-Durand J. Neurofibrillary tangles of Alzheimer’s disease: An immunohistochemical study. J. Submicrosc. Cytol. 1985;17:89–96. - PubMed
-
- Nishikawa T., Takahashi T., Nakamori M., Yamazaki Y., Kurashige T., Nagano Y., Nishida Y., Izumi Y., Matsumoto M. Phosphatidylinositol-4,5-bisphosphate is enriched in granulovacuolar degeneration bodies and neurofibrillary tangles. Neuropathol. Appl. Neurobiol. 2014;40:489–501. doi: 10.1111/nan.12056. - DOI - PMC - PubMed
-
- Morel E., Chamoun Z., Lasiecka Z.M., Chan R.B., Williamson R.L., Vetanovetz C., Dall’Armi C., Simoes S., Point Du Jour K.S., McCabe B.D., et al. Phosphatidylinositol-3-phosphate regulates sorting and processing of amyloid precursor protein through the endosomal system. Nat. Commun. 2013;4:2250. doi: 10.1038/ncomms3250. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- R01 AG042210/AG/NIA NIH HHS/United States
- U54 AG054345/AG/NIA NIH HHS/United States
- R01 AG039478/AG/NIA NIH HHS/United States
- U18 NS082140/NS/NINDS NIH HHS/United States
- U01 AG016976/AG/NIA NIH HHS/United States
- U24 AG041689/AG/NIA NIH HHS/United States
- R01 AG034374/AG/NIA NIH HHS/United States
- NA/Belgian FRS-FNRS
- NA/the Belgian Fondation Recherche Alzheimer/ Stichting Alzheimer Onderzoek
- T.0023.15/the Belgian Fonds de la Recherche Scientifique Médicale
- U01 AG046152/AG/NIA NIH HHS/United States
- NA/the Fund Aline (King Baudouin Foundation)
- R01 NS078009/NS/NINDS NIH HHS/United States
- NA/the Academic Medical Interdisciplinary Research (AMIR) Foundation
- R01 AG017917/AG/NIA NIH HHS/United States
- R01 AG036042/AG/NIA NIH HHS/United States
- P30 AG010161/AG/NIA NIH HHS/United States
- NA/the ULB Génicot Fund
- NA/Fondation Médicale Reine Elisabeth
- R01 AG034504/AG/NIA NIH HHS/United States
- R01 AG041232/AG/NIA NIH HHS/United States
- R01 AG036836/AG/NIA NIH HHS/United States
- R01 AG015819/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

