Functional Analysis of the PI3K/AKT/mTOR Pathway Inhibitor, Gedatolisib, Plus Fulvestrant with and Without Palbociclib in Breast Cancer Models
- PMID: 40565304
- PMCID: PMC12193243
- DOI: 10.3390/ijms26125844
Functional Analysis of the PI3K/AKT/mTOR Pathway Inhibitor, Gedatolisib, Plus Fulvestrant with and Without Palbociclib in Breast Cancer Models
Abstract
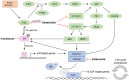
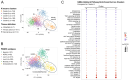
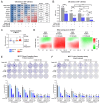
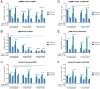
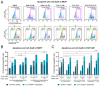
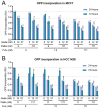
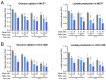
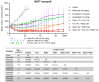
Treatment with endocrine therapy (ET) in combination with CDK4/6 inhibitors has improved the outcome of patients with hormone receptor (HR)+/HER2- advanced breast cancer (ABC), but most patients eventually experience disease progression. Since the PI3K-AKT-mTOR (PAM), estrogen receptor (ER), and cyclin-dependent kinase (CDK) pathways are interdependent drivers of HR+/HER2- breast cancer (BC), the simultaneous inhibition of these pathways is expected to enhance anti-tumor control. Here we investigated the molecular and cellular effects of gedatolisib, a multi-target kinase inhibitor of the PAM pathway currently being evaluated in Phase 3 clinical trials, combined with fulvestrant and/or palbociclib in BC cell models. We found that the gedatolisib/fulvestrant/palbociclib triplet inhibited BC cell growth significantly more than the single agents or the palbociclib/fulvestrant doublet, both in vitro and vivo. Specifically, the triplet combination counteracted adaptive responses associated with single drug treatment, such as the reactivation of the CDK-RB-E2F pathway after palbociclib treatment, and inhibited multiple cellular functions, such as cell cycle progression, cell survival, protein synthesis, and glucose metabolism. The triplet combination was effective in treatment-naïve BC cell lines as well as in cell lines adapted to palbociclib and/or fulvestrant, regardless of PIK3CA/PTEN genetic alterations. Our findings provide a mechanistic rationale for conducting clinical studies evaluating gedatolisib in combination with CDK4/6 inhibitors and ET in HR+/HER2- ABC.
Keywords: CDK4/6 inhibitors; PI3K-AKT-mTOR inhibitors; breast cancer; endocrine therapy; fulvestrant; gedatolisib; palbociclib.
Conflict of interest statement
A.B., S.R., A.S., L.D., M.S., S.S., A.M., J.M., and L.L. are all employed by and/or have ownership interest in Celcuity, Inc. A.D.L.F. was employed at Celcuity, Inc. at the time that these studies were performed and has ownership interest in Celcuity, Inc. A.S.M. is a consultant for Celcuity, Inc.
Figures

Similar articles
-
Extended follow-up of palbociclib with fulvestrant or letrozole for endocrine-sensitive, hormone receptor-positive/HER2-negative advanced breast cancer in the PARSIFAL trial.ESMO Open. 2025 Jul;10(7):105309. doi: 10.1016/j.esmoop.2025.105309. Epub 2025 Jun 17. ESMO Open. 2025. PMID: 40532361 Free PMC article. Clinical Trial.
-
Combined inhibition of CDK4/6 and AKT is highly effective against the luminal androgen receptor (LAR) subtype of triple negative breast cancer.Cancer Lett. 2024 Nov 1;604:217219. doi: 10.1016/j.canlet.2024.217219. Epub 2024 Sep 6. Cancer Lett. 2024. PMID: 39244005
-
Gedatolisib Combined with Palbociclib and Letrozole in Patients with No Prior Systemic Therapy for Hormone Receptor Positive, HER2 Negative Advanced Breast Cancer.Clin Cancer Res. 2025 Jul 25. doi: 10.1158/1078-0432.CCR-25-0992. Online ahead of print. Clin Cancer Res. 2025. PMID: 40711480
-
Comparison of palbociclib in combination with letrozole or fulvestrant with endocrine therapies for advanced/metastatic breast cancer: network meta-analysis.Curr Med Res Opin. 2017 Aug;33(8):1457-1466. doi: 10.1080/03007995.2017.1325730. Epub 2017 May 16. Curr Med Res Opin. 2017. PMID: 28463012
-
PROteolysis TArgeting Chimera (PROTAC) Estrogen Receptor Degraders for Treatment of Estrogen Receptor-Positive Advanced Breast Cancer.Target Oncol. 2025 May;20(3):431-444. doi: 10.1007/s11523-025-01137-5. Epub 2025 May 6. Target Oncol. 2025. PMID: 40327300 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

