The Impact of Non-Donor-Specific HLA Antibodies on Antibody-Mediated Rejection in Pediatric Kidney Transplant Recipients
- PMID: 40565332
- PMCID: PMC12192625
- DOI: 10.3390/ijms26125870
The Impact of Non-Donor-Specific HLA Antibodies on Antibody-Mediated Rejection in Pediatric Kidney Transplant Recipients
Abstract
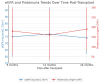
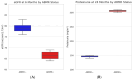
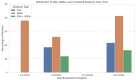
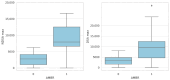
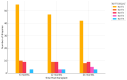
While the pathogenic role of donor-specific anti-HLA antibodies (DSAs) in long-term immune-mediated injury after kidney transplantation is well established, the clinical relevance of non-donor-specific antibodies (nDSAs), also detected in transplant recipients, remains a subject of debate. This retrospective study evaluated the prognostic value of nDSAs in 92 pediatric kidney transplant recipients (89.1%, 9.8%, and 1.1% for first, second, and third transplants, respectively) at the University Hospital of Padua between January 2015 and December 2022, investigating the association between antibody development and clinical outcomes, including graft function, rejection episodes, and viral infections. Clinical, immunological, virological, and histopathological data were collected at 6, 12, and 24 months post-transplant. Antibody prevalence increased over time, with nDSAs being more frequent than DSAs at all timepoints. The combined presence of DSAs and nDSAs significantly increased the risk of ABMR (HR = 45.10; p < 0.001). Isolated nDSAs and DSAs were also associated with an increased risk of ABMR (HR = 6.43 and 12.10, respectively), suggesting a synergistic alloimmune effect. Viral infections also emerged as relevant cofactors in humoral alloimmunity. EBV viremia and intrarenal Parvovirus B19 (PVB19) infection were significantly associated with ABMR, with PVB19 also correlating with nDSA formation. In conclusion, integrated immunological and virological monitoring may support risk stratification and guide individualized post-transplant management. Larger multicenter studies are warranted to define the long-term impact of nDSAs in pediatric kidney transplantation.
Keywords: antibody-mediated rejection; mean fluorescence intensity; non-donor-specific antibodies; pediatric kidney transplantation; viral infections.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Bachelet T., Martinez C., Del Bello A., Couzi L., Kejji S., Guidicelli G., Lepreux S., Visentin J., Congy-Jolivet N., Rostaing L., et al. Deleterious impact of donor-specific anti-HLA antibodies toward HLA-Cw and HLA-DP in kidney transplantation. Transplantation. 2016;100:159–166. doi: 10.1097/TP.0000000000000821. - DOI - PubMed
-
- Fichtner A., Gauché L., Süsal C., Tran T.H., Waldherr R., Krupka K., Guzzo I., Carraro A., Oh J., Zirngibl M., et al. Incidence, risk factors, management strategies, and outcomes of antibody-mediated rejection in pediatric kidney transplant recipients—A multicenter analysis of the Cooperative European Paediatric Renal Transplant Initiative (CERTAIN) Pediatr. Nephrol. 2025;40:491–503. doi: 10.1007/s00467-024-06487-2. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

