Elucidating Regulatory Mechanisms of Genes Involved in Pathobiology of Sjögren's Disease: Immunostimulation Using a Cell Culture Model
- PMID: 40565345
- PMCID: PMC12192667
- DOI: 10.3390/ijms26125881
Elucidating Regulatory Mechanisms of Genes Involved in Pathobiology of Sjögren's Disease: Immunostimulation Using a Cell Culture Model
Abstract
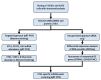
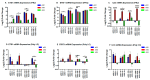
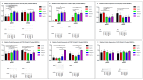
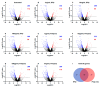
Sjögren's disease (SjD) is an autoimmune disease of exocrine tissues. Prior research has shown that ETS proto-oncogene 1 (ETS1), STAT1, and IL33 may contribute to the disease's pathology. However, the regulatory mechanisms of these genes remain poorly characterized. Our objective was to explore the mechanisms of SjD pathology and to identify dysfunctional regulators of these genes by immunostimulation of SjD and sicca relevant cell lines. We used immortalized salivary gland epithelial cell lines (iSGECs) from Sjögren's disease (pSS1) and sicca (nSS2) patients, previously developed in our lab, and control cell line A253 to dose with immunostimulants IFN-γ or poly(I:C) (0 to 1000 ng/mL and 0 to 1000 µg/mL, respectively) over a 72 h time course. Gene expression was determined using qRT-PCR delta-delta-CT method based on glyceraldehyde-3-phosphate dehydrogenase (GAPDH) for mRNA and U6 small nuclear RNA 1 (U6) for miRNA, using normalized relative fold changes 48 h post-immunostimulation. Protein expression was quantified 72 h post-stimulation by Western blotting. Reference-based RNA-seq of immunostimulated pSS1 and nSS2 cells was performed to characterize the reactome of genes conserved across all used doses. The expression of ETS1 and STAT1 protein was upregulated (p < 0.05) in IFN-γ-treated pSS1 and nSS2, as compared to A253 cells. IFN-γ-treated nSS2 cell showed significant IL33 upregulation. Also, IL33 had a correlated (p < 0.01) U-shaped response for low-mid-range doses for IFN-γ- and poly(I:C)-treated pSS1 cells. RNA-seq showed 175 conserved differentially expressed (DE) genes between nSS2 and pSS1 immunostimulated cells. Of these, 44 were shown to interact and 39 were more abundant (p < 0.05) in pSS1 cells. Western blotting demonstrated nSS2 cells expressing ETS1 uniformly across treatments compared to pSS1 cells, despite similar mRNA abundance. miR-145b and miR-193b were significantly under-expressed in IFN-γ-treated nSS2 cells compared to pSS1 cells (p < 0.01). ETS1 and IL33 showed disproportionate mRNA and protein abundances between immunostimulated Sjögren's disease-derived (pSS1), and sicca-derived (nSS2) cell lines. Such differences could be explained by higher levels of miR-145b and miR-193b present in pSS1 cells. Also, RNA-seq results suggested an increased sensitivity of pSS1 cells to immunostimulation. These results reflect current pathobiology aspects, confirming the relevance of immortalized salivary gland epithelial cell lines.
Keywords: ETS1; Interleukin-33; STAT1; Sjögren’s disease; gamma-IFN; iSGEC; miRNA; poly(I:C); regulation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Carsons S.E., Patel B.C. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2022. [(accessed on 1 August 2022)]. Sjogren Syndrome. Available online: https://www.ncbi.nlm.nih.gov/books/NBK431049/
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

