Retinal BMI1 Expression Preserves Photoreceptors in Sodium-Iodate-Induced Oxidative Stress Models
- PMID: 40565365
- PMCID: PMC12193584
- DOI: 10.3390/ijms26125907
Retinal BMI1 Expression Preserves Photoreceptors in Sodium-Iodate-Induced Oxidative Stress Models
Abstract
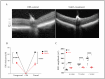
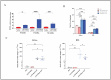
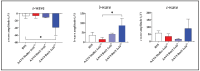
Dry age-related macular degeneration (AMD) is a leading cause of vision loss in individuals over 50, yet no approved therapies exist for early or intermediate stages of the disease. Oxidative stress is a central driver of retinal degeneration in AMD, and sodium iodate (NaIO3)-induced injury serves as a well-characterized model of oxidative damage to the retinal pigment epithelium (RPE) and photoreceptors. BMI1, a poly-comb group protein involved in DNA repair, mitochondrial function, and cellular renewal, has emerged as a promising therapeutic target for retinal neuroprotection. We evaluated the efficacy of AAV-mediated BMI1 gene delivery in murine models using two administration routes: subretinal (SR) and suprachoroidal (SC). AAV5.BMI1 (1 × 109 vg/eye) was delivered SR in Balb/c mice and evaluated at 4 and 15 weeks post-injection. AAV8.BMI1 (5 × 109 or 1 × 1010 vg/eye) was administered SC in C57BL/6 mice and assessed at 4 weeks. Control groups received BSS or AAV8.stuffer. Following NaIO3 exposure, retinal structure and function were analyzed by optical coherence tomography (OCT), electroretinography (ERG), histology, and molecular assays. SC delivery of AAV8.BMI1 achieved the highest levels of retinal BMI1 expression with no evidence of local or systemic toxicity. Treated eyes showed dose-dependent preservation of outer nuclear layer (ONL) thickness and significantly improved ERG responses indicating structural and functional protection. These findings support SC AAV.BMI1 gene therapy as a promising, minimally invasive, and translatable approach for early intervention in intermediate AMD.
Keywords: AAV gene therapy; BMI1; age-related macular degeneration; retina; retinal pigment epithelium; sodium iodate.
Conflict of interest statement
All authors are employed by the company Oculogenex Inc. Authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Wong W.L., Su X., Li X., Cheung C.M., Klein R., Cheng C.Y., Wong T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health. 2014;2:e106–e116. doi: 10.1016/S2214-109X(13)70145-1. - DOI - PubMed
-
- Holz F.G., Sadda S.R., Busbee B., Chew E.Y., Mitchell P., Tufail A., Brittain C., Ferrara D., Gray S., Honigberg L., et al. Efficacy and Safety of Lampalizumab for Geographic Atrophy Due to Age-Related Macular Degeneration: Chroma and Spectri Phase 3 Randomized Clinical Trials. JAMA Ophthalmol. 2018;136:666–677. doi: 10.1001/jamaophthalmol.2018.1544. - DOI - PMC - PubMed
-
- Khanani A.M., Hershberger V.S., Pieramici D.J., Khurana R.N., Brunstein F., Ma L., Maass K.F., Honigberg L.A., Tom I., Chen H., et al. Phase 1 Study of the Anti-HtrA1 Antibody-binding Fragment FHTR2163 in Geographic Atrophy Secondary to Age-related Macular Degeneration. Am. J. Ophthalmol. 2021;232:49–57. doi: 10.1016/j.ajo.2021.06.017. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

