Effects of C-Ring Structural Differences on the Inhibition of Nε-(Carboxyethyl)lysine in the Methylglyoxal-Lysine System by Flavonoids
- PMID: 40565377
- PMCID: PMC12193151
- DOI: 10.3390/ijms26125914
Effects of C-Ring Structural Differences on the Inhibition of Nε-(Carboxyethyl)lysine in the Methylglyoxal-Lysine System by Flavonoids
Abstract
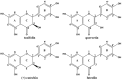
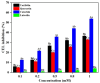
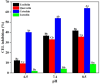
This study investigated the effects of taxifolin (Tax), quercetin (Que), (+)-catechin (Cat) and luteolin (Lute) on the advanced Maillard reaction stage in the methylglyoxal-lysine (MGO-Lys) system. Since the four flavonoids share identical A- and B-ring structures, the inhibitory effects and molecular mechanisms of flavonoids with different C-ring structures on Nε-(carboxyethyl)lysine (CEL) formation were revealed. The results demonstrated that Cat exhibited the best inhibitory effect on CEL with an inhibition rate of 53.78%, while Lute showed the lowest inhibition rate of 3.97%. The flavonoids (i.e., Tax, Que, Cat and Lute) inhibited the formation of non-fluorescent CEL, where hydroxylation at C3 on the C-ring favored the enhancement of the inhibitory effect of the flavonoids on CEL, while the C2-C3 double bond and the carbonyl group at the C4 position reduced their inhibitory ability. The alkaline environment favored the enhancement of the inhibition of CEL by Tax, Que, Cat and Lute. Notably, Tax, Que, Cat and Lute can inhibit CEL formation by competitively capturing MGO to form mono- or di-adducts and reducing lysine consumption. This study provides innovative strategies and a theoretical foundation for developing effective CEL inhibitors in food thermal processing.
Keywords: C-ring structures; Nε-(carboxyethyl)lysine (CEL); flavonoids; trapping methylglyoxal.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Influence of hydroxyl substitution on the inhibition of flavonoids in advanced glycation end-products formation in glucose-lysine-arginine Maillard reaction models.Food Res Int. 2025 Apr;207:116068. doi: 10.1016/j.foodres.2025.116068. Epub 2025 Feb 27. Food Res Int. 2025. PMID: 40086959
-
Proximal cysteine residues in proteins promote Nε-carboxyalkylation of lysine residues by α-dicarbonyl compounds.J Biol Chem. 2025 Apr;301(4):108377. doi: 10.1016/j.jbc.2025.108377. Epub 2025 Mar 4. J Biol Chem. 2025. PMID: 40049410 Free PMC article.
-
Effects of flavonol B-ring structures on the inhibitions of advanced glycation end products in bovine serum albumin-methylglyoxal models.Int J Biol Macromol. 2025 Aug;319(Pt 2):145378. doi: 10.1016/j.ijbiomac.2025.145378. Epub 2025 Jun 19. Int J Biol Macromol. 2025. PMID: 40543777
-
Measures implemented in the school setting to contain the COVID-19 pandemic.Cochrane Database Syst Rev. 2022 Jan 17;1(1):CD015029. doi: 10.1002/14651858.CD015029. Cochrane Database Syst Rev. 2022. Update in: Cochrane Database Syst Rev. 2024 May 2;5:CD015029. doi: 10.1002/14651858.CD015029.pub2. PMID: 35037252 Free PMC article. Updated.
-
The clinical effectiveness and cost-effectiveness of enzyme replacement therapy for Gaucher's disease: a systematic review.Health Technol Assess. 2006 Jul;10(24):iii-iv, ix-136. doi: 10.3310/hta10240. Health Technol Assess. 2006. PMID: 16796930
References
-
- Huang S., Dong X., Zhang Y., Chen Y., Yu Y., Huang M., Zheng Y. Formation of advanced glycation end products in raw and subsequently boiled broiler muscle: Biological variation and effects of postmortem ageing and storage. Food Sci. Hum. Wellness. 2022;11:255–262. doi: 10.1016/j.fshw.2021.11.012. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

