Heterorhabditis bacteriophora Extracellular Vesicles Alter the Innate Immune Signaling in Drosophila melanogaster
- PMID: 40565505
- PMCID: PMC12191515
- DOI: 10.3390/genes16060613
Heterorhabditis bacteriophora Extracellular Vesicles Alter the Innate Immune Signaling in Drosophila melanogaster
Abstract
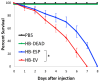
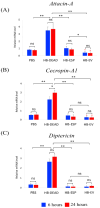
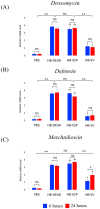
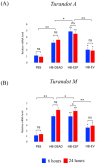
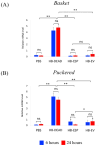
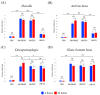
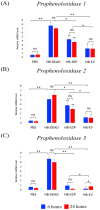
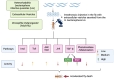
Background:Heterorhabditis bacteriophora entomopathogenic nematodes are commonly used in agricultural practices for the biological control of insect pests. These parasites are also used in basic research for unveiling the molecular basis of nematode parasitism in relation to the insect anti-nematode response. We have recently shown that H. bacteriophora excreted-secreted products reduce the expression of the antimicrobial peptide gene Diptericin in Drosophila melanogaster, which increases fly mortality due to enhanced propagation of the mutualistic bacteria Photorhabdus luminescens. However, the effect of entomopathogenic nematode extracellular vesicles (EVs) on the insect host defense remains unknown. Methods: Here, we injected adult flies with H. bacteriophora EVs and used quantitative RT-PCR together with gene-specific primers to analyze the activity of immune-related signaling pathways. Results: We found that H. bacteriophora EVs are lethal to Drosophila melanogaster, and they downregulate the expression of Attacin, Cecropin, and Prophenoloxidase 3 in adult flies. Conclusions: These findings build on previous knowledge and strengthen the notion that H. bacteriophora entomopathogenic nematodes release a variety of effector molecules to modify the insect's innate immune signaling. This information is important because it contributes toward clarifying the molecular interplay between entomopathogenic nematode components and the host's innate immune system.
Keywords: Drosophila; Heterorhabditis; immune gene expression; immune signaling; infection; innate immunity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
HETERORHABDITIS BACTERIOPHORA NEMATODES ARE SENSITIVE TO THE BACTERIAL PATHOGEN PHOTORHABDUS ASYMBIOTICA.J Parasitol. 2023 Jan 1;109(1):11-14. doi: 10.1645/22-55. J Parasitol. 2023. PMID: 36805240 Free PMC article.
-
Heterorhabditis bacteriophora symbiotic and axenic nematodes modify the Drosophila melanogaster larval microbiome.Front Microbiol. 2025 Jun 18;16:1598221. doi: 10.3389/fmicb.2025.1598221. eCollection 2025. Front Microbiol. 2025. PMID: 40606169 Free PMC article.
-
Activin and BMP Signaling Activity Affects Different Aspects of Host Anti-Nematode Immunity in Drosophila melanogaster.Front Immunol. 2021 Dec 22;12:795331. doi: 10.3389/fimmu.2021.795331. eCollection 2021. Front Immunol. 2021. PMID: 35003118 Free PMC article.
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

