Investigating the Sexual Dimorphism of Waist-to-Hip Ratio and Its Associations with Complex Traits
- PMID: 40565603
- PMCID: PMC12193181
- DOI: 10.3390/genes16060711
Investigating the Sexual Dimorphism of Waist-to-Hip Ratio and Its Associations with Complex Traits
Abstract
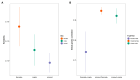
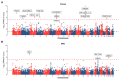
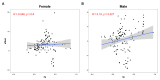
Background: Obesity significantly impacts disease burden, with waist-to-hip ratio (WHR) as a key obesity indicator, but the genetic and biological pathways underlying WHR, particularly its sex-specific differences, remain poorly understood. Methods: This study explored WHR's sexual dimorphism and its links to complex traits using cross-sectional surveys and genetic data from Giant and UK Biobank (UKB). We analyzed WHR heritability, performed tissue-specific transcriptome-wide association studies (TWAS) using FUSION, and conducted genetic correlation analyses with linkage disequilibrium score regression (LDSC) and Local Analysis of [co]Variant Association (LAVA). Polygenic scores (PGS) for WHR were constructed using the clumping and thresholding method (CT), and associations with complex traits were assessed via logistic or linear models. Results: The genetic analysis showed sex-specific heritability for WHR, with TWAS identifying female-specific (e.g., CCDC92) and male-specific (e.g., UQCC1) genes. Global genetic correlation analysis revealed sex-specific associations between WHR and 23 traits, while local analysis identified eight sex-specific loci across five diseases. Regression analysis highlighted sex-specific associations for 70 traits with WHR and 45 traits with WHR PGS, with stronger effects in females. Predictive models also performed better in females. Conclusions: This study underscores WHR's sexual dimorphism and its distinct associations with complex traits, offering insights into sex-specific biological differences, health management, and clinical advancements.
Keywords: PGS; WHR; genetic architecture; genetic correlation; sex-specific traits; sexual dimorphism.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
The genetics of low and high birthweight and their relationship with cardiometabolic disease.Diabetologia. 2025 Jul;68(7):1452-1462. doi: 10.1007/s00125-025-06420-8. Epub 2025 Apr 10. Diabetologia. 2025. PMID: 40210729 Free PMC article.
-
[Identifying genetic etiology of ischemic stroke based on pleiotropy of obesity related genes: A sibling study].Beijing Da Xue Xue Bao Yi Xue Ban. 2025 Jun 18;57(3):448-455. doi: 10.19723/j.issn.1671-167X.2025.03.007. Beijing Da Xue Xue Bao Yi Xue Ban. 2025. PMID: 40509821 Free PMC article. Chinese.
-
Discovery and fine-mapping of adiposity loci using high density imputation of genome-wide association studies in individuals of African ancestry: African Ancestry Anthropometry Genetics Consortium.PLoS Genet. 2017 Apr 21;13(4):e1006719. doi: 10.1371/journal.pgen.1006719. eCollection 2017 Apr. PLoS Genet. 2017. PMID: 28430825 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Abdominal obesity and gastroesophageal cancer risk: systematic review and meta-analysis of prospective studies.Biosci Rep. 2017 May 11;37(3):BSR20160474. doi: 10.1042/BSR20160474. Print 2017 Jun 30. Biosci Rep. 2017. PMID: 28336766 Free PMC article.
References
-
- Finucane M.M., Stevens G.A., Cowan M.J., Danaei G., Lin J.K., Paciorek C.J., Singh G.M., Gutierrez H.R., Lu Y., Bahalim A.N., et al. National, regional, and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. - DOI - PMC - PubMed
-
- Husain M.J., Datta B.K., Kostova D., Joseph K.T., Asma S., Richter P., Jaffe M.G., Kishore S.P. Access to Cardiovascular Disease and Hypertension Medicines in Developing Countries: An Analysis of Essential Medicine Lists, Price, Availability, and Affordability. J. Am. Heart Assoc. 2020;9:e015302. doi: 10.1161/JAHA.119.015302. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

