Food Allergenicity Evaluation Methods: Classification, Principle, and Applications
- PMID: 40565614
- PMCID: PMC12191692
- DOI: 10.3390/foods14122005
Food Allergenicity Evaluation Methods: Classification, Principle, and Applications
Abstract
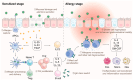
The incidence of food allergies is increasing annually, emerging as a significant global concern for food safety and public health. To prevent allergic reactions, patients are advised to avoid ingesting allergenic foods. However, completely avoiding contact with such foods in real life is often challenging. Therefore, the development of reliable allergenicity evaluation methods is essential to assist food allergy patients in avoiding exposure to allergenic foods. These evaluation methods include mass spectrometry-based methods, bioinformatics predictions, simulated digestion, enzyme-linked immunosorbent assays, cell-based models, and animal models. Each method operates on distinct principles and is suited for specific evaluation contexts. This review systematically summarizes the pathogenesis of food allergies and details the principles and practical applications of common allergenicity evaluation methods. By highlighting recent studies in food allergenicity evaluation, we aim to deepen the understanding of allergenicity assessment and offer an overview with perspectives on its enhancement.
Keywords: IgE-mediated allergy; allergenicity evaluation; epitope; food allergen; food allergy; immune responses.
Conflict of interest statement
All authors declare that they have no relevant conflicts of interest.
Figures


Similar articles
-
Skin care interventions in infants for preventing eczema and food allergy.Cochrane Database Syst Rev. 2022 Nov 14;11(11):CD013534. doi: 10.1002/14651858.CD013534.pub3. Cochrane Database Syst Rev. 2022. PMID: 36373988 Free PMC article.
-
Proposal of 0.5 mg of protein/100 g of processed food as threshold for voluntary declaration of food allergen traces in processed food-A first step in an initiative to better inform patients and avoid fatal allergic reactions: A GA²LEN position paper.Allergy. 2022 Jun;77(6):1736-1750. doi: 10.1111/all.15167. Epub 2021 Nov 24. Allergy. 2022. PMID: 34741557
-
Allergen-specific oral immunotherapy for peanut allergy.Cochrane Database Syst Rev. 2012 Sep 12;2012(9):CD009014. doi: 10.1002/14651858.CD009014.pub2. Cochrane Database Syst Rev. 2012. PMID: 22972130 Free PMC article.
-
Aerosol Measurements and Decadal Changes: The Role of Climatic Changes and How It Reflects in Respiratory Allergies and Asthma.Allergy. 2025 Jun;80(6):1613-1628. doi: 10.1111/all.16602. Epub 2025 May 31. Allergy. 2025. PMID: 40448467 Free PMC article. Review.
-
ImmunoCAP® ISAC and Microtest for multiplex allergen testing in people with difficult to manage allergic disease: a systematic review and cost analysis.Health Technol Assess. 2016 Sep;20(67):1-178. doi: 10.3310/hta20670. Health Technol Assess. 2016. PMID: 27623692 Free PMC article.
References
Publication types
Grants and funding
- 2024YFF1105905/National Key Research and Development Program of China
- 20232ACB205022/Key Project of Natural Science Foundation of Jiangxi Province, China
- 2022-PYXM-05/Jiangxi Nutrition and Health Management Medical Research Institute Cultivation Project
- NO.2022B0202030002/Special Project for Research and Development in Key areas of Guangdong Province
LinkOut - more resources
Full Text Sources

