Modulatory Effects of Tetraselmis chuii Gastrointestinal Digests on Human Colonic Microbiota
- PMID: 40565715
- PMCID: PMC12192011
- DOI: 10.3390/foods14122106
Modulatory Effects of Tetraselmis chuii Gastrointestinal Digests on Human Colonic Microbiota
Abstract
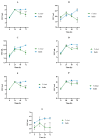
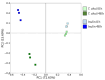
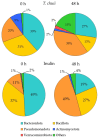
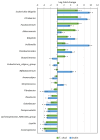
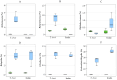
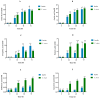
Tetraselmis chuii is a microalga commercialized because of its richness in health-beneficial molecules. Previous studies have profusely demonstrated the biological properties of compounds isolated from T. chuii, but data are not yet available on the impact that gastrointestinal digestion could exert. This article describes the passage of T. chuii through the gastrointestinal tract, combining the INFOGEST procedure and in vitro colonic fermentation to examine potential effects on the human colonic microflora composition and its metabolic activity. Microbial plate counting was conducted to determine the different groups of microorganisms. Amplification of the 16S ribosomal RNA gene was performed via polymerase chain reaction to examine in detail the main genera of bacteria, and its metabolic activity was evaluated by measuring of short-chain fatty acids (SCFAs) by gas chromatography. The presence of T. chuii modified the fecal microbiota. Although the evolution of lactic acid bacteria and Enterococcus spp. content during 72 h showed that the use of T. chuii, compared to fructopolysaccharides such as inulin, would not provide nutritional advantages, the microalgae extract contributed to a significant decrease in Clostridium, Staphylococcus, and Enterobacteriaceae. Furthermore, T. chuii increased the relative abundance of Akkermansia and Butyricimonas, genera considered highly beneficial. In correlation with the presence of these microorganisms, the results show that the presence of T. chuii favored the release of SCFA, such as acetic (20 mM), propionic (>5 mM), isovaleric (0.3 mM), isobutyric (0.15 mM), and, mainly, butyric (>2 mM), after 72 h colonic fermentation, being indicators of gut health. These findings suggest that T. chuii has potential as a functional ingredient for promoting health through its modulatory effects on the intestinal microbiota.
Keywords: Tetraselmis chuii; butyric acid; colonic fermentation; in vitro digestion; microalgae; microflora; short chain fatty acids.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
A systematic review and economic evaluation of epoetin alpha, epoetin beta and darbepoetin alpha in anaemia associated with cancer, especially that attributable to cancer treatment.Health Technol Assess. 2007 Apr;11(13):1-202, iii-iv. doi: 10.3310/hta11130. Health Technol Assess. 2007. PMID: 17408534
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
The clinical effectiveness and cost-effectiveness of enzyme replacement therapy for Gaucher's disease: a systematic review.Health Technol Assess. 2006 Jul;10(24):iii-iv, ix-136. doi: 10.3310/hta10240. Health Technol Assess. 2006. PMID: 16796930
References
-
- Barros de Medeiros V.P., da Costa W.K.A., da Silva R.T., Pimentel T.C., Magnani M. Microalgae as source of functional ingredients in new-generation foods: Challenges, technological effects, biological activity, and regulatory issues. Crit. Rev. Food Sci. Nutr. 2022;62:4929–4950. doi: 10.1080/10408398.2021.1879729. - DOI - PubMed
-
- Herrero Montarelo L.B., Gómez Vázquez M.D., Teruel Muñoz V.J. New regulation on novel foods in the European Union. AECOSAN Sci. Commun. J. 2015;27:123–132.
-
- AECOSAN Report of the scientific Committee of the Spanish Agency for Consumer Affairs, Food Safety and Nutrition (AECOSAN) on a request for initial assessment for marketing of the dried marine microalgae Tetraselmis chuii in food supplements under regulation (EC) no 258/97 on novel foods and novel food ingredients. AECOSAN Sci. Commun. J. 2017;25:11–21.
-
- FDA . Gras Notice Inventory. US Food and Drug Administration; Silver Spring, MD, USA: 2016. p. 26.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

