Effect of TGase Crosslinking on the Structure, Emulsification, and Gelling Properties of Soy Isolate Proteins
- PMID: 40565739
- PMCID: PMC12191487
- DOI: 10.3390/foods14122130
Effect of TGase Crosslinking on the Structure, Emulsification, and Gelling Properties of Soy Isolate Proteins
Abstract
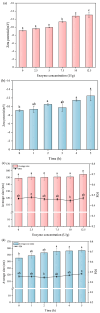
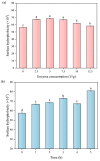
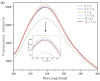
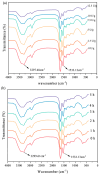
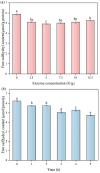
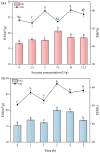
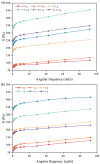
Soy isolate protein (SPI), as a high-quality plant protein source, is often processed into various soy products. In this study, the physicochemical properties of SPI treated with transglutaminase (TGase) were investigated in correlation with emulsification characteristics and rheological behavior. The polyacrylamide gel electrophoresis with sodium dodecyl sulfate (SDS-PAGE) and Fourier-transform infrared spectroscopy (FTIR) and endogenous fluorescence spectrum analysis results showed that TGase was able to promote the covalent binding of lysine and glutamine residues in SPI. The moderate pre-crosslinking treatment of TGase (5-7.5 U/g TGase pre-crosslinked for 2 h or 5 U/g TGase pre-crosslinked for 2-3 h) improved the emulsification and gel properties to varying degrees: the nanoparticle and emulsification performance increased by 24.35% and the storage modulus of the gel increased by 288%. Furthermore, the surface charge of SPI increased due to the crosslinking impact of TGase, indicating a considerable rise in the surface electrostatic potential. Simultaneously, the protein surface exhibited a substantial increase in hydrophobicity, while the level of free sulfhydryl groups reduced. These changes indicate that TGase enzymatic crosslinking could significantly improve the structural stability of nanoparticles by enhancing the generation efficiency of covalent bonds between protein molecules.
Keywords: conformational changes; crosslinking; properties; protein nanoparticles; transglutaminase.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Effects of Modification Technology on Soybean Protein Isolate: Physicochemical Characteristics, Protein Conformation, and Gel Properties.J Food Sci. 2025 Aug;90(8):e70459. doi: 10.1111/1750-3841.70459. J Food Sci. 2025. PMID: 40754688
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Effect of atmospheric-pressure cold plasma (ACP) treatment on the structure and function of WPI-EGCG complexes.Int J Biol Macromol. 2025 Jul;318(Pt 1):144854. doi: 10.1016/j.ijbiomac.2025.144854. Epub 2025 May 31. Int J Biol Macromol. 2025. PMID: 40456344
-
Eliciting adverse effects data from participants in clinical trials.Cochrane Database Syst Rev. 2018 Jan 16;1(1):MR000039. doi: 10.1002/14651858.MR000039.pub2. Cochrane Database Syst Rev. 2018. PMID: 29372930 Free PMC article.
References
-
- Peilong S., Weijun S., Zhengxun W., Sihong W., Ning X. Soy Protein Nanoparticles Prepared by Enzymatic Cross-Linking with Enhanced Emulsion Stability. Soft Matter. 2023;19:2099–2109. - PubMed
-
- Salunke P., Metzger L.E. Transglutaminase Crosslinked Milk Protein Concentrate and Micellar Casein Concentrate: Impact on the Functionality of Imitation Mozzarella Cheese Manufactured on a Small Scale Using a Rapid Visco Analyzer. Foods. 2024;13:2720. doi: 10.3390/foods13172720. - DOI - PMC - PubMed
-
- Zhu Q., Xu W., Zhang C., Gong J., Qin X., Zhang H., Liu G. Transglutaminase-Mediated Glycosylation Enhances the Physicochemical and Functional Properties of Ovalbumin. Food Hydrocoll. 2024;153:109992. doi: 10.1016/j.foodhyd.2024.109992. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

