Corneal Perforation as a Possible Ocular Adverse Event Caused by Cabozantinib: A Clinical Case and Brief Review
- PMID: 40565798
- PMCID: PMC12194347
- DOI: 10.3390/jcm14124052
Corneal Perforation as a Possible Ocular Adverse Event Caused by Cabozantinib: A Clinical Case and Brief Review
Abstract
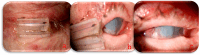
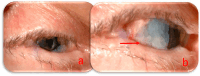
Background: Cabozantinib is a Vascular Endothelial Growth Factor Receptor Tyrosine Kinase Inhibitor (VEGFR-TKI). These drugs are employed as therapy for several malignancies. In detail, Cabozantinib has demonstrated its efficacy against several malignancies. On the other hand, Cabozantinib and other VEGFR-TKIs can be responsible for various adverse events (AEs), in particular hepatic and dermatological AEs. Methods: To date, limited data are available in the literature regarding ocular AEs due to therapy with these drugs. In this regard, one case of corneal perforation during treatment with a VEGFR-TKI, Regorafenib, has been reported, while there are no data about Cabozantinib. In this paper, we present another clinical case of corneal perforation in a patient affected by advanced RCC and treated with Cabozantinib as a second-line therapy. The patient started Cabozantinib at the dosage of 60 mg/die although it was necessary to apply some dose reductions because of grade 2 AEs (according to CTCAE v6.0), such as asthenia, diarrhea, dysgeusia, and loss of appetite. Results: After approximately 15 months of treatment, the patient began to experience pain and vision loss in the right eye. A diagnosis of corneal perforation was made, followed by medical and surgical treatment. As regards the etiology of this pathology, all other possible causes were excluded, including a history of ocular disease, contact trauma, exposure to damaging agents (e.g., chemical agents and prolonged use of drugs such as topical NSAIDs), infections, or dry eye. Therefore, we hypothesized a correlation with Cabozantinib's mechanisms of action and paused its administration. Conclusions: Cabozantinib may alter the ocular environment due to a lack of or imbalance in growth factors in the tear film, with a reduction in corneal epithelium proliferation. This condition might cause dry eye and a delay in corneal healing. Therefore, particular importance should be placed on ophthalmologic surveillance during treatment with these drugs in patients who develop ocular symptoms. Further in vitro and in vivo studies are necessary to deepen the knowledge about VEGFR-TKI-mediated ocular AEs.
Keywords: Cabozantinib; corneal perforation; ocular adverse event; renal cell carcinoma; vascular endothelial growth factor receptor.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Schlumberger M., Elisei R., Müller S., Schöffski P., Brose M., Shah M., Licitra L., Krajewska J., Kreissl M.C., Niederle B., et al. Overall Survival Analysis of EXAM, a Phase III Trial of Cabozantinib in Patients with Radiographically Progressive Medullary Thyroid Carcinoma. Ann. Oncol. 2017;28:2813–2819. doi: 10.1093/annonc/mdx479. - DOI - PMC - PubMed
-
- Choueiri T.K., Escudier B., Powles T., Tannir N.M., Mainwaring P.N., Rini B.I., Hammers H.J., Donskov F., Roth B.J., Peltola K., et al. Cabozantinib versus Everolimus in Advanced Renal Cell Carcinoma (METEOR): Final Results from a Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2016;17:917–927. doi: 10.1016/S1470-2045(16)30107-3. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

