Focusing on Selinexor for Holding and Bridging Prior to CAR-T in Relapsed/Refractory Multiple Myeloma
- PMID: 40565816
- PMCID: PMC12194457
- DOI: 10.3390/jcm14124071
Focusing on Selinexor for Holding and Bridging Prior to CAR-T in Relapsed/Refractory Multiple Myeloma
Abstract
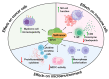
Background: The remarkable efficacy of B-cell maturation antigen (BCMA)-directed chimeric antigen receptor T-cell therapy (CAR-T) has had a significant impact on treatment strategies for relapsed/refractory multiple myeloma (RRMM). However, response durability remains a concern, necessitating the optimization of CAR-T procedures. Therapies preceding CAR-T therapy are crucial for disease control and preserving T-cell fitness. Methods: This review summarizes the evidence supporting the potential of selinexor-based regimens as holding or bridging therapy with preclinical research, demonstrating selinexor's ability to foster an anti-inflammatory tumor microenvironment. Results: Selinexor enhances CD8+ T-lymphocyte and NK cell activation, re-polarizes macrophages, and inhibits immunosuppressive cells. Bone marrow samples from patients in clinical studies show that selinexor increases CD8 and granzyme B expression in T-cells. Selinexor also disrupts NK cell inhibition, enhances anti-tumor activity, and reduces pro-inflammatory cytokines. Selinexor may upregulate BCMA expression and increase myeloma cell immunogenicity. Real-world data suggests selinexor as bridging therapy does not compromise CAR-T outcomes and may even improve them. Conclusions: Overall, the evidence indicates selinexor's potential to optimize CAR-T outcomes, warranting further investigation as a holding or bridging therapy for CAR-T.
Keywords: BCMA; CAR-T; T-cell exhaustion; bridging; selinexor.
Conflict of interest statement
J.K. reports Honoraria: J&J, GPCR Therapeutics, Prothena; Consulting or Advisory Role: J&J, Kite, Legend Biotech. D.S. reports Consulting or Advisory Role: Sanofi, GlaxoSmithKline, Bristol Myers Squibb/Celgene, Janssen, AbbVie, Pfizer, Arcellx, BiolineRx, AstraZeneca; Research Funding: Pfizer. A.R. reports Consulting: Johnson & Johnson, Bristol Myers Squibb, Karyopharm, Adaptive, and Sanofi. T.M. (Thomas Martin) reports Research Funding: Sanofi, Janssen, Amgen, and BMS; Consulting: GSK, Roche, and Pfizer. T.K. reports Karyopharm Therapeutics employment. T.M. (Tomer Mark) reports Karyopharm Therapeutics employment. M.B. reports Independent review committee: Parexel; Advisory Boards: Janssen Research, BMS/Celgene, Sanofi-Genzyme, Pfizer, Prothena, AbbVie; Consultancy: Pfizer, AbbVie, J&J. The funder’s role in the manuscript was limited to the input from the employees listed above.
Figures
References
-
- Rytlewski J., Madduri D., Fuller J., Campbell T.B., Mashadi-Hossein A., Thompson E.G., Jiang Y., Martin N., Sangurdekar D., Finney O., et al. Effects of Prior Alkylating Therapies on Preinfusion Patient Characteristics and Starting Material for CAR T Cell Product Manufacturing in Late-Line Multiple Myeloma. Blood. 2020;136:7–8. doi: 10.1182/blood-2020-134369. - DOI
-
- Firestone R.S., McAvoy D., Shekarkhand T., Serrano E., Hamadeh I., Wang A., Zhu M., Qin W.G., Patel D., Tan C.R., et al. CD8 Effector T Cells Enhance Teclistamab Response in BCMA-Exposed and -Naïve Multiple Myeloma. Blood Adv. 2023;8:1600–1611. doi: 10.1182/bloodadvances.2023011225. - DOI - PMC - PubMed
-
- Afrough A., Hashmi H., Hansen D.K., Sidana S., Ahn C., Peres L.C., Dima D., Freeman C.L., Puglianini O.C., Kocoglu M.H., et al. Real-World Impact of Bridging Therapy on Outcomes of Ide-Cel for Myeloma in the U.S. Myeloma Immunotherapy Consortium. Blood Cancer J. 2024;14:63. doi: 10.1038/s41408-024-00993-0. - DOI - PMC - PubMed
-
- Costa L.J., Banerjee R., Mian H., Weisel K., Bal S., Derman B.A., Htut M.M., Nagarajan C., Rodriguez C., Richter J., et al. International Myeloma Working Group Immunotherapy Committee Recommendation on Sequencing Immunotherapy for Treatment of Multiple Myeloma. Leukemia. 2025;39:543–554. doi: 10.1038/s41375-024-02482-6. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

