Lamotrigine Therapy: Relation Between Treatment of Bipolar Affective Disorder and Incidence of Stevens-Johnson Syndrome-A Narrative Review of the Existing Literature
- PMID: 40565849
- PMCID: PMC12194526
- DOI: 10.3390/jcm14124103
Lamotrigine Therapy: Relation Between Treatment of Bipolar Affective Disorder and Incidence of Stevens-Johnson Syndrome-A Narrative Review of the Existing Literature
Abstract
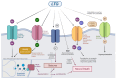
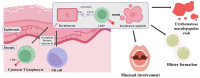
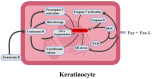
Lamotrigine is the drug of choice for the treatment of depressive episodes in bipolar disorder (BD). Despite its generally favorable tolerability profile, lamotrigine use is associated with a risk of Cutaneous Adverse Drug Reactions (cADRs), including Stevens-Johnson Syndrome (SJS) and Lyell's syndrome, also known as toxic epidermal necrolysis (TEN). Genetic markers HLA and, in particular, HLA-B 15:02 and HLA-A 31:01 are crucial in predicting individuals' susceptibility to developing the symptoms. The symptoms are triggered by type IV hypersensitivity developing because of CTL and NK cell activation, leading to keratinocyte apoptosis, epidermal necrosis and skin detachment. The exact pharmacotherapy that should be widely utilized in treating affected patients has not yet been established. New therapies including JAK inhibitors or cyclosporine show potential in improving outcomes by reducing mortality and enhancing the period of recovery. Key factors in preventing cADRs may include adequate patient observation, gradual titration of the patient's dose, and reduction of risk factors through screening for HLA polymorphisms. When the initial symptoms of cADR are identified, it is imperative to make an immediate decision to discontinue treatment, as this can significantly reduce the risk of progression to SJS/TEN and systemic complications. The purpose of this review is to identify a significant correlation between lamotrigine use in BD and the occurrence of SJS by showing the risk factors, neuropharmacological mechanisms, immune response and correctness of pharmacotherapy.
Keywords: Stevens–Johnson syndrome; bipolar affective disorder; cutaneous adverse drug reactions; hypersensitivity reactions; lamotrigine; mood stabilizers.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Systemic interventions for treatment of Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS/TEN overlap syndrome.Cochrane Database Syst Rev. 2022 Mar 11;3(3):CD013130. doi: 10.1002/14651858.CD013130.pub2. Cochrane Database Syst Rev. 2022. PMID: 35274741 Free PMC article.
-
Lamotrigine Emerging as a Driver of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: An 8-Year Retrospective Study.Burns. 2024 Nov;50(8):2114-2123. doi: 10.1016/j.burns.2024.07.006. Epub 2024 Jul 20. Burns. 2024. PMID: 39127578
-
A systematic review and economic model of the clinical effectiveness and cost-effectiveness of interventions for preventing relapse in people with bipolar disorder.Health Technol Assess. 2007 Oct;11(39):iii-iv, ix-206. doi: 10.3310/hta11390. Health Technol Assess. 2007. PMID: 17903393
-
Role of human leukocyte antigen in anti-epileptic drugs-induced Stevens-Johnson Syndrome/toxic epidermal necrolysis: A meta-analysis.Seizure. 2022 Nov;102:36-50. doi: 10.1016/j.seizure.2022.09.011. Epub 2022 Sep 17. Seizure. 2022. PMID: 36183454
-
A systematic review of case-control studies of cytokines in blister fluid and skin tissue of patients with Stevens Johnson syndrome and toxic epidermal necrolysis.Australas J Dermatol. 2024 Sep;65(6):491-504. doi: 10.1111/ajd.14329. Epub 2024 Jun 3. Australas J Dermatol. 2024. PMID: 38831709
Cited by
-
Epigenetics and Gut Microbiota in the Pathogenesis and Treatment of Bipolar Disorder (BD).Cells. 2025 Jul 18;14(14):1104. doi: 10.3390/cells14141104. Cells. 2025. PMID: 40710357 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

