Emerging Age-Specific Therapeutic Approaches for Dry Eye Disease
- PMID: 40565894
- PMCID: PMC12194506
- DOI: 10.3390/jcm14124147
Emerging Age-Specific Therapeutic Approaches for Dry Eye Disease
Abstract
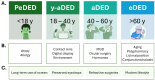
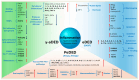
Dry eye disease (DED) is a common, multifactorial disorder of the ocular surface. Although DED can affect individuals at any age, its prevalence, clinical manifestations, underlying mechanisms, and optimal management strategies differ considerably across the lifespan. In children, symptoms are frequently associated with atopy and allergic disorders and environmental factors, whereas in young adults, digital device usage and contact lens wear are the predominant contributors. In older adults, systemic diseases and polypharmacy significantly elevate the risk of DED. Across all age groups, tear film instability, decreased tear production, and chronic inflammation are central pathogenic features. Key tear biomarkers, such as pro-inflammatory cytokines, have been widely linked to disease development. Cathepsin S and tumor necrosis factor-alpha have recently been implicated in age-related DED. A nuanced understanding of these age-related differences is crucial for improving diagnostic accuracy and tailoring interventions to specific patient populations. This review synthesizes current evidence on DED across age groups, focusing on prevalence, risk factors, pathophysiology, molecular mechanisms, coexisting conditions, biomarkers, and treatment options. Finally, it highlights critical unmet clinical needs in the management of age-related DED.
Keywords: age targeted; biomarkers; children; dry eye disease; elderly; ocular surface; tears; treatment; young adults.
Conflict of interest statement
T.S-C. is employee of FAES FARMA (Spain). II.H. declares no potential conflict of interest.
Figures
References
-
- Baudouin C., Messmer E.M., Aragona P., Geerling G., Akova Y.A., Benítez-Del-Castillo J., Boboridis K.G., Merayo-Lloves J., Rolando M., Labetoulle M. Revisiting the vicious circle of dry eye disease: A focus on the pathophysiology of meibomian gland dysfunction. Br. J. Ophthalmol. 2016;100:300–306. doi: 10.1136/bjophthalmol-2015-307415. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

