Safety and Performance of Postmarketing Breast Implants: An Integrated Review with Technovigilance Data
- PMID: 40565910
- PMCID: PMC12194552
- DOI: 10.3390/jcm14124164
Safety and Performance of Postmarketing Breast Implants: An Integrated Review with Technovigilance Data
Abstract
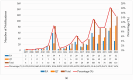
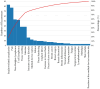
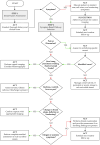
Background/Objectives: Breast implants are widely used in reconstructive surgeries, as well as in cosmetic procedures, to enhance or restore breast shape and volume. With advances in techniques and materials, these devices have become safer and more effective over the years. Nevertheless, complications such as capsular contracture, rupture, infections, or other types of malignancies (BIA-SCC). This study evaluated the postmarketing safety and performance of implants via technovigilance data and a review of scientific studies. Methods: The research analyzed publications from the BVS, PubMed, Embase, and ClinicalTrials databases from between 2007 and 2023 (15 years), in addition to reports registered in the Notivisa system during the same period. Results: A total of 113 studies were identified, 15 of which were selected for the final analysis, which revealed that capsular contracture, seroma, infection, and rupture were the most common complications. In the Notivisa system, 786 reports were found, including 397 technical complaints and 389 adverse events, with pain, infections, and lymphoma among the most frequently reported issues. Conclusions: These findings highlight the importance of continuous surveillance to identify risks and promote improvements in the quality and safety of breast implants, ensuring patient well-being. As a practical contribution, a clinical decision-making algorithm was proposed to support healthcare professionals in the early identification and management of implant-related complications.
Keywords: breast implants; breasts; health surveillance; medical device safety.
Conflict of interest statement
There are no conflicts of interest.
Figures


Similar articles
-
Different types of implants for reconstructive breast surgery.Cochrane Database Syst Rev. 2016 May 16;2016(5):CD010895. doi: 10.1002/14651858.CD010895.pub2. Cochrane Database Syst Rev. 2016. PMID: 27182693 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Cephalomedullary nails versus extramedullary implants for extracapsular hip fractures in older adults.Cochrane Database Syst Rev. 2022 Jan 26;1(1):CD000093. doi: 10.1002/14651858.CD000093.pub6. Cochrane Database Syst Rev. 2022. PMID: 35080771 Free PMC article.
References
-
- Sudha T.S., Varghese A.M., Kumar Z.N., Sasanka K.K., Hari T.S., Thangaraju P. Medical Devices. CRC Press; Boca Raton, FL, USA: 2022. Implants and Prosthetics; pp. 94–125.
-
- Non-Active Surgical Implants: Mammary Implants: Particular Requirements. International Organization for Standardization; Geneva, Switzerland: 2018. p. 41.
-
- Anvisa . Resolução de Diretoria Colegiada—RDC nº 665, de 30 de Março de 2022. Dispõe Sobre as Boas Práticas de Fabricação de Produtos Médicos e Produtos para Diagnóstico de Uso In Vitro. Agência Nacional de Vigilância Sanitária; Brasília, Brazil: 2022. p. 26.
LinkOut - more resources
Full Text Sources
Research Materials

