Induction or Adjuvant Chemotherapy in Patients with Locally Advanced Nasopharyngeal Cancer Receiving Chemoradiotherapy? A Turkish Oncology Group Study
- PMID: 40565933
- PMCID: PMC12194331
- DOI: 10.3390/jcm14124189
Induction or Adjuvant Chemotherapy in Patients with Locally Advanced Nasopharyngeal Cancer Receiving Chemoradiotherapy? A Turkish Oncology Group Study
Abstract
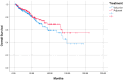
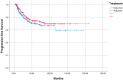
Background/Objectives: In this retrospective study, we aimed to compare the efficacy, survival, and toxicity results of induction (IC) or adjuvant (AC) treatment with chemoradiotherapy (CRT) in locally advanced nasopharyngeal cancer (NPC). Methods: A total of 405 patients from 22 different centres in Turkey, belonging to the Turkish Oncology Group (TOG), was included. The primary endpoints were overall survival (OS) and progression-free survival (PFS), and the secondary endpoints were safety and toxicity. Results: The median age of the patients included in the study was 49 (18.2-91.5) years. In total, 298 (73.6%) of the patients were male. Of the 405 patients, 258 (63.7%) received IC and 147 (36.3%) received AC treatment. When OS and PFS analyses were performed in terms of age, gender, T and N stages, pathological features, and treatments received, no effect of any variable on prognosis was observed. For the overall group, the median estimated OS was 137.3 months (the Kaplan-Meier statistical method could not reach the 95% confidence interval [CI]). For the IC group, the median estimated survival was 137.3 months (95% CI: 111.4-163.3), whereas the Kaplan-Meier statistical method could not estimate survival for the AC group. No statistically significant difference was observed between IC and AC groups in terms of OS (p = 0.209) or PFS (p = 0.248). Grade 3-4 side effects were observed in 12% of patients in the IC group and 29.9% of patients in the AC group. Treatment was discontinued due to toxicity in 5 patients (1.9%) in the IC group and 18 patients (12.2%) in the AC group. Conclusion: No difference in OS or PFS was observed between AC and IC treatments. More grade 3-4 side effects were observed in the AC-treated group and early discontinuation rate was higher.
Keywords: adjuvant; chemotherapy; induction; nasopharyngeal cancer.
Conflict of interest statement
None of the authors has any significant direct or indirect commercial or financial incentives associated with the publication of the article.
Figures
Similar articles
-
Optimisation of chemotherapy and radiotherapy for untreated Hodgkin lymphoma patients with respect to second malignant neoplasms, overall and progression-free survival: individual participant data analysis.Cochrane Database Syst Rev. 2017 Sep 13;9(9):CD008814. doi: 10.1002/14651858.CD008814.pub2. Cochrane Database Syst Rev. 2017. PMID: 28901021 Free PMC article.
-
Treatment options for progression or recurrence of glioblastoma: a network meta-analysis.Cochrane Database Syst Rev. 2021 May 4;5(1):CD013579. doi: 10.1002/14651858.CD013579.pub2. Cochrane Database Syst Rev. 2021. PMID: 34559423 Free PMC article.
-
The use of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer: systematic review and economic evaluation.Health Technol Assess. 2008 May;12(15):iii-ix, xi-162. doi: 10.3310/hta12150. Health Technol Assess. 2008. PMID: 18462574
-
Hysterectomy with radiotherapy or chemotherapy or both for women with locally advanced cervical cancer.Cochrane Database Syst Rev. 2022 Aug 22;8(8):CD010260. doi: 10.1002/14651858.CD010260.pub3. Cochrane Database Syst Rev. 2022. PMID: 35994243 Free PMC article.
-
Comparison of first-line chemotherapy including escalated BEACOPP versus chemotherapy including ABVD for people with early unfavourable or advanced stage Hodgkin lymphoma.Cochrane Database Syst Rev. 2017 May 25;5(5):CD007941. doi: 10.1002/14651858.CD007941.pub3. Cochrane Database Syst Rev. 2017. PMID: 28541603 Free PMC article.
References
-
- Juarez-Vignon Whaley J.J., Afkhami M., Onyshchenko M., Massarelli E., Sampath S., Amini A., Bell D., Villaflor V.M. Recurrent/Metastatic Nasopharyngeal Carcinoma Treatment from Present to Future: Where Are We and Where Are We Heading? Curr. Treat Options Oncol. 2023;24:1138–1166. doi: 10.1007/s11864-023-01101-3. - DOI - PMC - PubMed
-
- Bossi P., Chan A.T., Licitra L., Trama A., Orlandi E., Hui E.P., Halámková J., Mattheis S., Baujat B., Hardillo J., et al. Nasopharyngeal carcinoma: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up(†) Ann. Oncol. 2021;32:452–465. doi: 10.1016/j.annonc.2020.12.007. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials