Induction or Adjuvant Chemotherapy in Patients with Locally Advanced Nasopharyngeal Cancer Receiving Chemoradiotherapy? A Turkish Oncology Group Study
- PMID: 40565933
- PMCID: PMC12194331
- DOI: 10.3390/jcm14124189
Induction or Adjuvant Chemotherapy in Patients with Locally Advanced Nasopharyngeal Cancer Receiving Chemoradiotherapy? A Turkish Oncology Group Study
Abstract
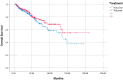
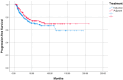
Background/Objectives: In this retrospective study, we aimed to compare the efficacy, survival, and toxicity results of induction (IC) or adjuvant (AC) treatment with chemoradiotherapy (CRT) in locally advanced nasopharyngeal cancer (NPC). Methods: A total of 405 patients from 22 different centres in Turkey, belonging to the Turkish Oncology Group (TOG), was included. The primary endpoints were overall survival (OS) and progression-free survival (PFS), and the secondary endpoints were safety and toxicity. Results: The median age of the patients included in the study was 49 (18.2-91.5) years. In total, 298 (73.6%) of the patients were male. Of the 405 patients, 258 (63.7%) received IC and 147 (36.3%) received AC treatment. When OS and PFS analyses were performed in terms of age, gender, T and N stages, pathological features, and treatments received, no effect of any variable on prognosis was observed. For the overall group, the median estimated OS was 137.3 months (the Kaplan-Meier statistical method could not reach the 95% confidence interval [CI]). For the IC group, the median estimated survival was 137.3 months (95% CI: 111.4-163.3), whereas the Kaplan-Meier statistical method could not estimate survival for the AC group. No statistically significant difference was observed between IC and AC groups in terms of OS (p = 0.209) or PFS (p = 0.248). Grade 3-4 side effects were observed in 12% of patients in the IC group and 29.9% of patients in the AC group. Treatment was discontinued due to toxicity in 5 patients (1.9%) in the IC group and 18 patients (12.2%) in the AC group. Conclusion: No difference in OS or PFS was observed between AC and IC treatments. More grade 3-4 side effects were observed in the AC-treated group and early discontinuation rate was higher.
Keywords: adjuvant; chemotherapy; induction; nasopharyngeal cancer.
Conflict of interest statement
None of the authors has any significant direct or indirect commercial or financial incentives associated with the publication of the article.
Figures
References
-
- Juarez-Vignon Whaley J.J., Afkhami M., Onyshchenko M., Massarelli E., Sampath S., Amini A., Bell D., Villaflor V.M. Recurrent/Metastatic Nasopharyngeal Carcinoma Treatment from Present to Future: Where Are We and Where Are We Heading? Curr. Treat Options Oncol. 2023;24:1138–1166. doi: 10.1007/s11864-023-01101-3. - DOI - PMC - PubMed
-
- Bossi P., Chan A.T., Licitra L., Trama A., Orlandi E., Hui E.P., Halámková J., Mattheis S., Baujat B., Hardillo J., et al. Nasopharyngeal carcinoma: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up(†) Ann. Oncol. 2021;32:452–465. doi: 10.1016/j.annonc.2020.12.007. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials