Incidence of Sepsis-Induced Coagulopathy (INSIC) Trial: Study Protocol of a Combined Retrospective and Prospective, Multicenter, International, Cross-Sectional, Longitudinal, and Epidemiological Observational Trial
- PMID: 40565967
- PMCID: PMC12194450
- DOI: 10.3390/jcm14124222
Incidence of Sepsis-Induced Coagulopathy (INSIC) Trial: Study Protocol of a Combined Retrospective and Prospective, Multicenter, International, Cross-Sectional, Longitudinal, and Epidemiological Observational Trial
Abstract
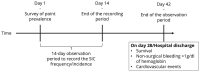
Background/Objectives: Sepsis and septic shock are the most severe forms of infection. Due to the intensive cross-talk of the coagulation and immune system, coagulopathies regularly occur in sepsis. The International Society on Thrombosis and Hemostasis refers to these coagulation abnormalities as sepsis-induced coagulopathies (SICs). The presence of SICs can be assessed using the SIC score. In parallel, a score for "sepsis-associated coagulopathy" (SAC) was introduced that, in contrast to the SIC score, allows coagulopathy to be classified according to its severity. In the past, multicenter, randomized controlled trials have repeatedly failed to prove the efficacy of specific therapeutic measures targeting SICs or SACs. This could potentially be explained by insufficient knowledge about the prevalence and incidence of SIC and the rate of spontaneous recovery. The Incidence of Sepsis-Induced Coagulopathy (INSIC) trial is intended to address this problem. Methods: The aim of the INSIC trial is to measure the incidence and prevalence of SIC-in addition to the rate of spontaneous SIC recoveries-during the first days of sepsis treatment to provide a solid base of data for future interventional trials. We aim to include all patients with sepsis treated in any one of 150 participating intensive care units in 100 hospitals located in Austria, Luxembourg, and Germany to determine the prevalence of SIC (on day 1 of the study period as well as on three selected days in the fourth quarter of each year from 2019 to 2024). SIC incidence will be assessed over a 14-day period starting on 10 March 2025. Secondary endpoints are 28-day survival and the occurrence of severe thromboembolic events and bleeding. In addition, each of the aforementioned outcome parameters will be assessed for correlation with the severity classification of the SAC score. Conclusions: The INSIC trial is the first study to determine the prevalence as well as the incidence of SIC, to prospectively examine the course of SIC longitudinally over a 14-day period, and to determine the rate of spontaneous recoveries within 72 h under standard treatment in a large cohort of patients. This information will provide a sound basis for future studies.
Keywords: incidence; prevalence; sepsis; sepsis-induced coagulopathy; septic shock.
Conflict of interest statement
T. Schmoch, M. Bauer, F. Bloos, S. Frank, J. Gregorius, D. I. Radke, H. Bogatsch, and J. Briegel declare that they have no conflicts of interest. T. Brenner reports that he has received honoraria for lectures or sitting on advisory boards from the following organizations: Baxter Deutschland GmbH, Schöchl medical education GmbH (Germany), and CSL Behring GmbH (Germany). T. Brenner reports that he also received honoraria for advisory board and consulting activity from BAYER AG (Germany). P. Möhnle reports that he has received lecture fees and reimbursements for conference fees, travel, and accommodation expenses from Bayer (Germany), Biotest (Germany), CSL Behring (Germany), NovoNordisk (Germany), Pfizer (Germany), Octapharma (Germany), Roche (Germany), Shire/Takeda, and SOBI (Germany), and honoraria for advisory board and/or consulting activity from Alexion Pharma (Germany), Astra Zeneca (Germany), CSL Behring (Germany), SOBI (Germany), and Takeda (Germany). M. A. Weigand reports that he has received lecture fees from GE Healthcare (Germany), Gilead (Germany), Köhler Chemie (Germany), MSD Sharp & Dohme (Germany), Pfizer Pharma (Germany), and Boehringer Ingelheim (Germany), and has been a member of advisory boards at B. Braun (Germany), Gilead (Germany), MSD Sharp & Dohme (Germany), and Shionogi (Germany). P. Meybohm and/or the Department of Anaesthesiology, Intensive Care, Emergency and Pain Medicine, University Hospital Würzburg (Würzburg, Germany), received research grants from the German Research Foundation (ME 3559/1-1, ME 3559/3-1), BMBF (01KG1815), and BMG (ZMVI1-2520DAT10E); grants from B. Braun Melsungen, CSL Behring, Fresenius Kabi, and Vifor Pharma for the implementation of Frankfurt’s Patient Blood Management Program; and honoraria for scientific lectures from Biotest AG, Vifor Pharma, CSL Behring, and Pharmacosmos. Maximilian Dietrich reports that he has received lecture fees from CSL Behring GmbH (Germany).
Figures

References
-
- Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.D., Coopersmith C.M., et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. - DOI - PMC - PubMed
-
- Schmoch T., Weigand M., Möhnle P., Briegel J., Brenner T. Sepsis-Induzierte Koagulopathie: Zu häufig Missachtete Komplikation der Sepsis. INTENSIV-News [Internet] 2023. [(accessed on 8 June 2025)]. Available online: https://medicom.cc/de/publikationen/intensiv-news/202306/entries/03-Seps....
-
- Taylor F.B., Toh C.H., Hoots W.K., Wada H., Levi M., Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH) Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb. Haemost. 2001;86:1327–1330. doi: 10.1055/s-0037-1616068. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

