Clinical Management of Cerebral Amyloid Angiopathy
- PMID: 40566003
- PMCID: PMC12194787
- DOI: 10.3390/jcm14124259
Clinical Management of Cerebral Amyloid Angiopathy
Abstract
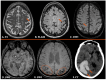
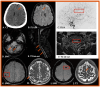
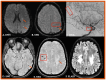
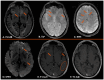
Background: Cerebral amyloid angiopathy (CAA) represents a progressive cerebrovascular disorder, characterized by aberrant accumulation of beta-amyloid isoforms in cortical and leptomeningeal vessel walls of cerebrum and cerebellum. Methods: We sought to investigate the clinical manifestations, current different diagnostic tools, various therapeutic strategies and most uncommon subtypes of the disease. Results: The vast majority of CAA remains sporadic, with increasing prevalence with age and very frequent coexistence with Alzheimer's disease. Clinically, CAA can present with spontaneous lobar intracerebral hemorrhage, transient focal neurologic episodes attributed to convexity subarachnoid hemorrhage or cortical superficial siderosis, and progressive cognitive decline leading to dementia. Inflammatory CAA subtype should be recognized early and treated promptly so that better functional outcomes may be achieved. Moreover, genetic and iatrogenic CAA forms are rare, yet increasingly recognized during the last years. Therapeutic management remains challenging for clinicians, especially when markers indicative of higher bleeding risk are present. A targeted therapy does not currently exist. However, various clinical trials are in progress, focusing on offering new promising insights into the disease treatment. Conclusions: This review aims to deepen our understanding of CAA diagnosis and therapeutic approach but also summarizes current evidence on the most uncommon subtypes of this cerebral small-vessel disease.
Keywords: CAA-ri; amyloid PET; amyloid-β; brain MRI; cerebral amyloid angiopathy; cerebral microbleeds; cerebrospinal fluid biomarkers; cortical superficial siderosis; treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Koemans E.A., Chhatwal J.P., van Veluw S.J., van Etten E.S., van Osch M.J.P., van Walderveen M.A.A., Sohrabi H.R., Kozberg M.G., Shirzadi Z., Terwindt G.M., et al. Progression of cerebral amyloid angiopathy: A pathophysiological framework. Lancet Neurol. 2023;22:632–642. doi: 10.1016/S1474-4422(23)00114-X. - DOI - PubMed
-
- Malhotra K., Zompola C., Theodorou A., Katsanos A.H., Shoamanesh A., Gupta H., Beshara S., Goyal N., Chang J., Tayal A.H., et al. Prevalence, Characteristics, and Outcomes of Undetermined Intracerebral Hemorrhage: A Systematic Review and Meta-Analysis. Stroke. 2021;52:3602–3612. doi: 10.1161/STROKEAHA.120.031471. - DOI - PubMed
-
- Xiong L., Boulouis G., Charidimou A., Roongpiboonsopit D., Jessel M.J., Pasi M., Reijmer Y.D., Fotiadis P., Ayres A., Merrill E., et al. Dementia incidence and predictors in cerebral amyloid angiopathy patients without intracerebral hemorrhage. J. Cereb. Blood Flow Metab. 2018;38:241–249. doi: 10.1177/0271678X17700435. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

