The Interplay Between Obesity and Type 2 Diabetes: Common Pathophysiological Mechanisms Contributing to Telomere Shortening
- PMID: 40566527
- PMCID: PMC12194111
- DOI: 10.3390/life15060873
The Interplay Between Obesity and Type 2 Diabetes: Common Pathophysiological Mechanisms Contributing to Telomere Shortening
Abstract
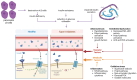
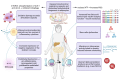
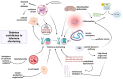
The worldwide prevalence of obesity continues to increase, representing a serious public health issue due to associated comorbidities. Obesity is associated with type 2 diabetes mellitus (T2D), which shares similar pathophysiological mechanisms. In both conditions, oxidative stress, inflammation, mitochondrial dysfunction, abnormal adipose tissue function, and senescence are observed, ultimately leading to insulin resistance. In both cases, hypertrophic adipose tissue is associated with telomere shortening. Elucidating the mechanisms underlying telomere shortening in obesity and diabetes may be crucial for deepening our understanding of these pathologies, with the ultimate aim of its translational implications. Several studies have shown that telomere shortening is present in patients with metabolic disorders, emphasizing its prognostic value for the onset and progression of these diseases. From this perspective, this article highlights the importance of telomere biology, which can aid in developing new therapeutic options for metabolic disorders.
Keywords: age-related diseases; aging biomarkers; chronic inflammation; insulin resistance; obesity; oxidative stress; senescence; telomere shortening; telomeres; type 2 diabetes mellitus.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Carbamazepine versus phenytoin monotherapy for epilepsy: an individual participant data review.Cochrane Database Syst Rev. 2017 Feb 27;2(2):CD001911. doi: 10.1002/14651858.CD001911.pub3. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2019 Jul 18;7:CD001911. doi: 10.1002/14651858.CD001911.pub4. PMID: 28240353 Free PMC article. Updated.
-
Unravelling the role of telomere shortening with ageing and their potential association with diabetes, cancer, and related lifestyle factors.Tissue Cell. 2022 Dec;79:101925. doi: 10.1016/j.tice.2022.101925. Epub 2022 Sep 12. Tissue Cell. 2022. PMID: 36137363
-
Cumulative methotrexate dose is not associated with liver fibrosis in patients with a history of moderate-to-severe psoriasis.Br J Dermatol. 2024 Jul 16;191(2):275-283. doi: 10.1093/bjd/ljae069. Br J Dermatol. 2024. PMID: 38366967
-
Continuous subcutaneous insulin infusion versus multiple daily injections of insulin for pregnant women with diabetes.Cochrane Database Syst Rev. 2016 Jun 7;2016(6):CD005542. doi: 10.1002/14651858.CD005542.pub3. Cochrane Database Syst Rev. 2016. PMID: 27272351 Free PMC article.
-
Inhaled mannitol for cystic fibrosis.Cochrane Database Syst Rev. 2018 Feb 9;2(2):CD008649. doi: 10.1002/14651858.CD008649.pub3. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2020 May 1;5:CD008649. doi: 10.1002/14651858.CD008649.pub4. PMID: 29424930 Free PMC article. Updated.
References
-
- WHO Ageing and Health. [(accessed on 25 May 2025)]. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health.
Publication types
LinkOut - more resources
Full Text Sources

