Voltage-Gated Ion Channels in Neuropathic Pain Signaling
- PMID: 40566541
- PMCID: PMC12194696
- DOI: 10.3390/life15060888
Voltage-Gated Ion Channels in Neuropathic Pain Signaling
Abstract
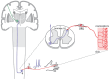
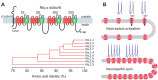
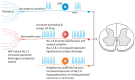
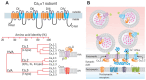
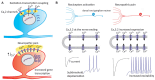
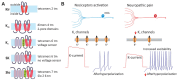
Neuropathic pain is a chronic and debilitating disorder of the somatosensory system that affects a significant proportion of the population and is characterized by abnormal responses such as hyperalgesia and allodynia. Voltage-gated ion channels, including sodium (NaV), calcium (CaV), and potassium (KV) channels, play a pivotal role in modulating neuronal excitability and pain signal transmission following nerve injury. This review intends to provide a comprehensive analysis of the molecular and cellular mechanisms by which dysregulation in the expression, localization, and function of specific NaV channel subtypes (mainly NaV1.7 and NaV1.8) and their auxiliary subunits contributes to aberrant neuronal activation, the generation of ectopic discharges, and sensitization in neuropathic pain. Likewise, special emphasis is placed on the crucial role of CaV channels, particularly CaV2.2 and the auxiliary subunit CaVα2δ, whose overexpression increases calcium influx, neurotransmitter release, and neuronal hyperexcitability, thus maintaining persistent pain states. Furthermore, KV channels (particularly KV7 channels) function as brakes on neuronal excitability, and their dysregulation facilitates the development and maintenance of neuropathic pain. Therefore, targeting specific KV channel subtypes to restore their function is also a promising therapeutic strategy for alleviating neuropathic pain symptoms. On the other hand, recent advances in the development of small molecules as selective modulators or inhibitors targeting voltage-gated ion channels are also discussed. These agents have improved efficacy and safety profiles in preclinical and clinical studies by attenuating pathophysiological channel activity and restoring neuronal function. This review seeks to contribute to guiding future research and drug development toward more effective mechanism-based treatments by discussing the molecular mechanisms underlying neuropathic pain and highlighting translational therapeutic opportunities.
Keywords: CaV channels; KV channels; NaV channels; PROTACs; calcium channels; neuropathic pain; potassium channels; sodium channels; voltage-gated ion channels.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

