Combining 31P-1H Cross Polarization With Magnetization Transfer: A Novel Approach for Myelin Investigation
- PMID: 40566864
- PMCID: PMC12198715
- DOI: 10.1002/nbm.70086
Combining 31P-1H Cross Polarization With Magnetization Transfer: A Novel Approach for Myelin Investigation
Abstract
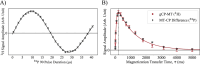
MRI is a crucial tool for studying white matter, which is primarily composed of myelin, a phospholipid-rich sheath surrounding nerve fibers. Myelin damage leads to disrupted neurological function, which is a prominent feature in neurodegenerative diseases like multiple sclerosis. Current MRI techniques for detecting myelin use hydrogen nuclei (1H) exclusively to generate contrast. Phosphorus (31P) is highly concentrated in myelin phospholipids relative to other brain structures. Due to sensitivity of the anisotropic chemical shifts and dipolar couplings to structure and dynamics, 31P may provide richer and more specific ways to probe the myelin bilayers. Key experiments aimed at developing MRI compatible probes of myelin 31P are demonstrated. First, a solid-state NMR technique, cross polarization (CP) is compared with single pulse excitation in white matter. The 1H-31P CP spectrum retains the morphology sensitive 31P powder pattern of the single-pulse spectrum, but lacks the aqueous 31P peak. Second, by combining magnetization transfer (MT) with CP, we observe bi-directional polarization exchange between myelin 31P and surrounding water, apparently proceeding through a unique 1H pool that is distinct from the 1H that typically dominates MT. Pulsed magnetic field gradients are used to isolate magnetization that originates from myelin 31P and transferred to aqueous 1H. This small signal, approximately 1/68,000 that of the 1H water signal, could offer access to structural and dynamic information from the membrane/water interface not previously available. This two-step transfer process opens new possibilities for understanding myelin and white matter disease and injury. These proof-of-principle findings may have broad implications for both basic neuroscience and clinical imaging. By leveraging 31P as a myelin probe, this approach offers a novel tool for studying myelin and could aid in detection and treatment monitoring of white matter disease and injury. Future work will investigate the translation of this technique to MRI.
Keywords: NMR; cross polarization; magnetization transfer; myelin; phosphorus; white matter.
© 2025 The Author(s). NMR in Biomedicine published by John Wiley & Sons Ltd.
Figures



Similar articles
-
Translating state-of-the-art spinal cord MRI techniques to clinical use: A systematic review of clinical studies utilizing DTI, MT, MWF, MRS, and fMRI.Neuroimage Clin. 2015 Dec 4;10:192-238. doi: 10.1016/j.nicl.2015.11.019. eCollection 2016. Neuroimage Clin. 2015. PMID: 26862478 Free PMC article.
-
Magnetic resonance perfusion for differentiating low-grade from high-grade gliomas at first presentation.Cochrane Database Syst Rev. 2018 Jan 22;1(1):CD011551. doi: 10.1002/14651858.CD011551.pub2. Cochrane Database Syst Rev. 2018. PMID: 29357120 Free PMC article.
-
Factors that influence parents' and informal caregivers' views and practices regarding routine childhood vaccination: a qualitative evidence synthesis.Cochrane Database Syst Rev. 2021 Oct 27;10(10):CD013265. doi: 10.1002/14651858.CD013265.pub2. Cochrane Database Syst Rev. 2021. PMID: 34706066 Free PMC article.
-
Quantitative magnetization transfer and g-ratio imaging of white matter myelin in early psychotic spectrum disorders.Mol Psychiatry. 2025 Jun;30(6):2739-2747. doi: 10.1038/s41380-024-02883-0. Epub 2025 Jan 8. Mol Psychiatry. 2025. PMID: 39779900 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
-
- Filley C., “White Matter Structure and Function,” in The Behavioral Neurology of White Matter, 2nd ed. (Oxford University Press, Inc, 2012), 23–43.
MeSH terms
Substances
Grants and funding
- RGPIN-2022-04807/Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grants
- RGPIN-2019-05245/Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grants
- RGPIN-2015-04513/Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grants
- NSERC
- MS Canada Doctoral Studentship Award
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

