STOX1 Isoform A Promotes Proliferation and Progression of Hepatocellular Carcinoma by Dual Mechanisms of Transcriptionally Upregulation of Cyclin B1 and Activation of ROS-Dependent PTEN/AKT1 Signaling
- PMID: 40567110
- PMCID: PMC12198656
- DOI: 10.1002/cam4.70958
STOX1 Isoform A Promotes Proliferation and Progression of Hepatocellular Carcinoma by Dual Mechanisms of Transcriptionally Upregulation of Cyclin B1 and Activation of ROS-Dependent PTEN/AKT1 Signaling
Abstract
Background: Dysregulation of transcription factors is one of the most common factors for the pathogenesis of hepatocellular carcinoma (HCC). To the best of our knowledge, no study has yet investigated the clinical significance and functional role of STOX1 in HCC.
Methods: Real-time PCR, Western blotting and immunohistochemistry were performed to examine the expression of STOX1-A in HCC specimens. Animal experiment in vivo and functional cell assays in vitro were used to investigate the tumorigenic and proliferative ability of HCC cells. Luciferase and ROS assays were depolyed to investigate the molecular mechanisms underlying the biologic role of STOX1-A in HCC.
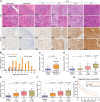
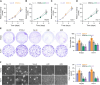
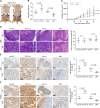
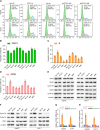
Results: In this study, we report that STOX1 isoform A (STOX1-A) is significantly upregulated in HCC tissues, and elevated STOX1-A levels are associated with poorer overall survival and progression-free survival in HCC patients. Functional assays demonstrated that STOX1-A upregulation promotes, whereas its silencing suppresses, HCC cell proliferation and growth both in vitro and in vivo. Mechanistic investigations revealed a dual mechanism by which STOX1-A drives HCC progression. First, STOX1-A transcriptionally upregulates cyclin B1, promoting cell proliferation. Second, it activates the AKT1 signaling pathway through reactive oxygen species (ROS)-mediated deactivation of PTEN. Furthermore, a positive correlation between STOX1-A expression and the levels of cyclin B1 and phosphorylated AKT1 (p-AKT1 Ser473) was observed in clinical HCC samples.
Conclusion: Our findings identify a novel dual mechanism by which STOX1-A promotes HCC proliferation and growth, offering potential avenues for the development of anti-tumor therapeutic strategies targeting STOX1-A in HCC.
Keywords: AKT1 signaling pathway; STOX1 isoform A; cell proliferation and cell cycle; hepatocellular carcinoma; reactive oxygen species.
© 2025 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Bray F., Ferlay J., Soerjomataram I., Siegel R. L., Torre L. A., and Jemal A., “Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries,” CA: A Cancer Journal for Clinicians 68, no. 6 (2018): 394–424. - PubMed
-
- Llovet J. M., Kelley R. K., Villanueva A., et al., “Hepatocellular Carcinoma,” Nature Reviews. Disease Primers 7 (2021): 6. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

