RNF122 targets STING for ubiquitination at residues K95, K117, and K155 to regulate antiviral responses in a teleost fish
- PMID: 40567163
- PMCID: PMC12464376
- DOI: 10.24272/j.issn.2095-8137.2025.033
RNF122 targets STING for ubiquitination at residues K95, K117, and K155 to regulate antiviral responses in a teleost fish
Abstract
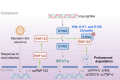
Ring finger protein 122 (RNF122), an E3 ubiquitin ligase, orchestrates antiviral immune responses in mammals by targeting retinoic acid-inducible gene 1 and melanoma differentiation-associated gene 5 for ubiquitination. However, its functional relevance in teleosts has yet to be clearly defined, particularly regarding the identification of substrate-specific regulatory sites. This study characterized RNF122 from mandarin fish ( Siniperca chuatsi), termed scRNF122, and investigated its regulatory impact on stimulator of interferon genes (STING)-mediated antiviral signaling. Results showed that scRNF122 expression was up-regulated in response to mandarin fish ranavirus (MRV) infection, and its overexpression suppressed scSTING-mediated interferon (IFN) production and enhanced MRV replication. Co-immunoprecipitation confirmed a direct interaction between scRNF122 and scSTING. Functional assays demonstrated that scRNF122 facilitated scSTING degradation through the ubiquitin-proteasome pathway, a process impeded by MG132 treatment. Ubiquitination analyses of various scSTING mutants revealed that scRNF122 catalyzed scSTING ubiquitination at K95, K117, and K155 residues. Moreover, scRNF122 significantly impaired scSTING-dependent antiviral responses by engaging negative regulatory elements within the signaling cascade. Overall, scRNF122 was identified as a negative modulator of STING-mediated IFN signaling in mandarin fish, diminishing STING-dependent antiviral activity and promoting its degradation via the ubiquitin-proteasome pathway at lysine residues K95, K117, and K155. These findings provide mechanistic insight into the post-translational control of STING in teleosts and establish a foundation for future investigations into antiviral immune regulation.
环指蛋白122(RNF122)作为一种E3泛素连接酶,在哺乳动物中通过泛素化黄酸诱导基因1和黑色素瘤分化相关基因5调控抗病毒免疫应答。然而,关于RNF122在硬骨鱼类中的功能认知仍较匮乏,尤其是其底物特异性调控位点的鉴定尚不明确。该研究以鳜( Siniperca chuatsi)RNF122( scRNF122)为对象,探究其在鳜干扰素基因刺激因子( scSTING)介导的抗病毒免疫通路中的作用机制,并揭示 scSTING蛋白上的关键调控位点。结果表明:病毒感染细胞中 scRNF122的表达模式响应鳜蛙虹彩病毒(MRV)感染;过表达 scRNF122可抑制 scSTING介导的干扰素(IFN)生成并促进MRV复制;免疫共沉淀实验证实 scRNF122与 scSTING存在蛋白质间的相互作用。进一步实验发现, scRNF122通过促进 scSTING的降解发挥抑制作用,而蛋白酶体抑制剂MG132可阻断该降解过程。通过对不同 scSTING突变体进行泛素化实验分析,发现 scRNF122催化 scSTING第95、117和155位赖氨酸(K95、K117、K155)的泛素化修饰。此外,该研究还证实, scRNF122能显著削弱 scSTING介导的抗MRV感染的作用。该研究阐明了 scRNF122蛋白通过泛素-蛋白酶体途径在三个关键赖氨酸位点(K95、K117、K155)介导STING降解,从而负调控STING介导的IFN信号通路并削弱其抗病毒功能。该发现为深入阐明硬骨鱼类STING介导的抗病毒先天性免疫调控机制提供重要理论依据。.
Keywords: Innate immunity; Interferon; RNF122; STING; Ubiquitination.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
-
- Benson DA, Cavanaugh M, Clark K, et al GenBank. Nucleic Acids Research. 2013;41(D1):D36–D42.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
