Advancing Design Strategy of PROTACs for Cancer Therapy
- PMID: 40567248
- PMCID: PMC12188103
- DOI: 10.1002/mco2.70258
Advancing Design Strategy of PROTACs for Cancer Therapy
Abstract
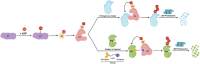
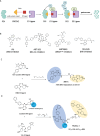
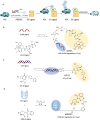
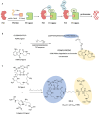
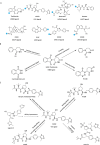
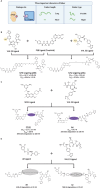
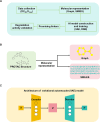
Proteolysis targeting chimeras (PROTACs) have emerged as a groundbreaking class of anticancer therapeutics. These bifunctional molecules harness the endogenous ubiquitin-proteasome system to facilitate the degradation of targeted proteins of interest (POIs). Notably, the clinical translation of PROTACs has gained substantial momentum, with many PROTAC candidates targeting various cancers currently undergoing clinical trials (Phase I-III). However, the rational design of high-efficacy PROTAC compounds remains a significant challenge. In this review, we presented a comprehensive overview of POI ligands, E3 ligands, and their interconnected linkers in PROTAC design, including their generation, structural optimization, and contribution to degradation efficiency and selectivity. Particularly, we analyzed the distinct preferences of various types of POI ligands (small molecule, nucleic acid, and peptide) toward specific targets. Furthermore, we emphasized the significant role of artificial intelligence technology in PROTAC design, including POI/E3 ligands discovery and linkers generation or optimization. We also summarized the applications and challenges of PROTACs in cancer therapy. Finally, we discussed the future development of PROTAC by combining multidisciplinary technologies and novel modalities for cancer therapy. Overall, this review aims to provide valuable insights for advancing PROTAC design strategies for cancer therapy.
Keywords: E3 ligand; POI ligand; artificial intelligence; cancer therapy; linker design; proteolysis targeting chimeras (PROTACs).
© 2025 The Author(s). MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
All authors declare no conflicts of interest.
Figures
References
-
- Glickman M. H. and Ciechanover A., “The Ubiquitin‐proteasome Proteolytic Pathway: Destruction for the Sake of Construction,” Physiological Reviews 82, no. 2 (2002): 373–428. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
