The efficacy and safety of kappa opioid receptor (KOR) agonists in patients with uraemic pruritus: a systematic review and network meta-analysis
- PMID: 40567495
- PMCID: PMC12188196
- DOI: 10.1093/ckj/sfaf131
The efficacy and safety of kappa opioid receptor (KOR) agonists in patients with uraemic pruritus: a systematic review and network meta-analysis
Abstract
Background: Uraemic pruritus (UP) is an increasingly significant health burden. However, current treatments are often unsatisfactory and associated with numerous adverse reactions. Recently, several large randomized controlled trials (RCTs) have confirmed that kappa opioid receptor (KOR) agonists, which target the endogenous opioid system, are effective in controlling symptoms. We compared the efficacy and safety of currently available KOR agonists for the treatment of UP.
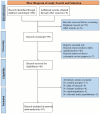
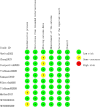
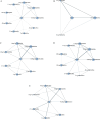
Methods: We conducted a systematic review and network meta-analysis (NMA) of RCTs to assess the efficacy and safety of KOR agonists in patients with UP. The primary outcomes were pruritus-related scales and adverse events. Two independent reviewers evaluated RCTs for eligibility and extracted relevant data, with discrepancies resolved by consensus or a third reviewer. We utilized a fixed effects model within a Bayesian framework for the NMA. Dichotomous variables were presented as risk ratios (RRs) and continuous variables were merged using standardized mean differences. Statistical analyses were performed using R 4.2.3 and JAGS 4.3.0. The risk of bias was assessed using the RoB 2 tool and the certainty of findings was rated according to Grading of Recommendations Assessment, Development and Evaluation criteria. The study protocol was registered on PROSPERO (CRD42020169955).
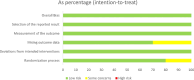
Results: Ten studies with 2483 participants were included. Concerning the primary endpoints, difelikefalin at doses of 0.25 µg/kg, 0.5 µg/kg, 1.0 µg/kg and 1.5 µg/kg, nalfurafine at 2.5 µg and 5 µg and nalbuphine at 120 mg were significantly effective in reducing itching severity compared with placebo. For the secondary endpoint, all four doses of difelikefalin were associated with higher rates of adverse events compared with placebo, while other interventions showed rates comparable to those of placebo and did not present statistically significant differences.
Conclusion: In summary, difelikefalin at doses of 0.25 µg/kg and 0.5 µg/kg, along with nalfurafine at 0.25 µg/kg and 0.5 µg/kg, can be considered recommended therapeutic options for UP treatment.
Keywords: kappa opioid receptor; network meta-analysis; uraemic pruritus.
© The Author(s) 2025. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
LinkOut - more resources
Full Text Sources

