KRAS and NRF2 drive metabolic reprogramming in pancreatic cancer cells: the influence of oxidative and nitrosatice stress
- PMID: 40567499
- PMCID: PMC12187747
- DOI: 10.3389/fcell.2025.1547582
KRAS and NRF2 drive metabolic reprogramming in pancreatic cancer cells: the influence of oxidative and nitrosatice stress
Abstract
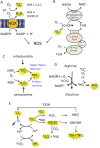
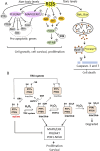
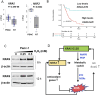
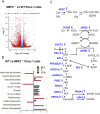
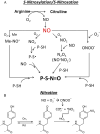
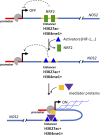
Cancer cells are subject to metabolic reprogramming, which leads to a sustained production of reactive oxygen species (ROS). Increased oxidative stress contributes to genomic instability and promotes malignant transformation. To counteract excessive ROS levels, cells activate nuclear factor erythroid 2-related factor 2 (NRF2), a key regulator of redox homeostasis that coordinates the transcription of a wide range of antioxidant and cytoprotective genes. This review examines the metabolic adaptations controlled by the KRAS-NRF2 axis under oxidative stress conditions. In addition, we highlight a novel function of NRF2 in regulating the expression of NOS2 by binding to a DNA enhancer element, thereby modulating the production of reactive nitrogen species (RNS). Finally, we discuss novel molecular strategies aimed at disrupting adaptive antioxidant responses in cancer cells and provide insights into combinatorial therapeutic approaches targeting redox balance in cancer.
Keywords: KRAS; NOS2; NRF2; PDAC; RNS; ROS; metabolic reprogramming.
Copyright © 2025 Rapozzi, Comuzzi, Di Giorgio and Xodo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
Similar articles
-
The Central Role of NRF2 in Cancer Metabolism and Redox Signaling: Novel Insights into Crosstalk with ER Stress and Therapeutic Modulation.Cell Biochem Biophys. 2025 Jul 15. doi: 10.1007/s12013-025-01821-3. Online ahead of print. Cell Biochem Biophys. 2025. PMID: 40665070 Review.
-
The role of nuclear factor erythroid 2-related factor 2 (NRF2) in normal and pathological pregnancy: A systematic review.Am J Reprod Immunol. 2021 Dec;86(6):e13496. doi: 10.1111/aji.13496. Epub 2021 Sep 19. Am J Reprod Immunol. 2021. PMID: 34467607
-
A systematic review of p53 regulation of oxidative stress in skeletal muscle.Redox Rep. 2018 Dec;23(1):100-117. doi: 10.1080/13510002.2017.1416773. Epub 2018 Jan 3. Redox Rep. 2018. PMID: 29298131 Free PMC article.
-
NRF2 maintains redox balance via ME1 and NRF2 inhibitor synergizes with venetoclax in NPM1-mutated acute myeloid leukemia.Cancer Metab. 2025 Jun 18;13(1):32. doi: 10.1186/s40170-025-00401-6. Cancer Metab. 2025. PMID: 40533864 Free PMC article.
-
Oncogenic stress response mechanisms as new therapeutic targets in cancer treatment: A review.Medicine (Baltimore). 2025 Jun 13;104(24):e42857. doi: 10.1097/MD.0000000000042857. Medicine (Baltimore). 2025. PMID: 40527836 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

