Structural determination of MCOFs: status, challenges and perspectives
- PMID: 40567549
- PMCID: PMC12186186
- DOI: 10.1039/d5sc03670d
Structural determination of MCOFs: status, challenges and perspectives
Abstract
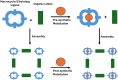
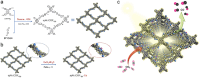
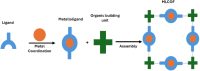
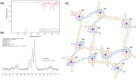
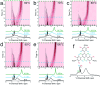
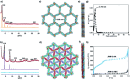
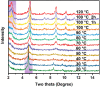
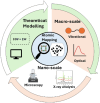
Porous materials have many promising characteristics, including tuneable chemical and optical properties, modifiable porosities and large surface areas. Long-range order frameworks have effective evaluation methods as a result of their crystallinities and in this context, nanoscale analysis, namely single-crystal X-ray diffraction, is a particularly useful approach for optimising the structure-property relationships. Metal-covalent organic frameworks (MCOFs), synthesised by incorporating a metal complex into a stable covalent organic framework (COF) backbone, have shown considerable promise for a variety of applications. Nonetheless, their wide-scale implementation remains hindered due to difficulties in structurally mapping them; their typically reduced crystallinities result in major challenges for their structural determination. By classifying MCOFs as metalated COFs (MeCOFs) and metalloligand COFs (MLCOFs), the characterisation of these lower crystallinity frameworks can be carried out according to their distinctive architecture using a combination of complementary structural analysis techniques. This perspective highlights examples of a synergistic approach to the structural elucidation of MLCOFs to overcome obstacles related to their crystalline nature, generating an atomic map through a combination of nano and macroscale characterisation procedures supported by theoretical modelling tools. The effective use of structural characterisation methods is considered in this perspective, which can reveal key information regarding the structure-activity relationships as they relate to MLCOFs.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures







References
Publication types
LinkOut - more resources
Full Text Sources

